Periodic system and the structure of the atom briefly. Periodic law and the periodic system of chemical elements of DI Mendeleev on the basis of ideas about the structure of the atom
1. wording of the periodic law
DI Mendeleev in the light of the theory of the structure of the atom.
The discovery of the periodic law and the development of the periodic system of chemical elements DI Mendeleev were the pinnacle of the development of chemistry in the XIX century. A vast amount of knowledge about the properties of the 63 elements, known by that time, was put in orderly order.
DI Mendeleev believed that the main characteristic of the elements are their atomic weights, and in 1869 he formulated the periodic law for the first time.
The properties of simple bodies, as well as the form and properties of compounds of elements, are in periodic dependence on the size of the atomic weights of the elements.
Mendeleev divided the entire series of elements arranged in ascending order of atomic masses into periods, within which the properties of the elements change sequentially, placing the periods so as to single out similar elements.
However, despite the enormous significance of such a conclusion, the periodic law and the Mendeleev system represented only a brilliant generalization of the facts, and their physical meaning remained unclear for a long time. Only as a result of the development of the physics of the 20th century — the discovery of the electron, radioactivity, the development of the theory of the structure of the atom — a young, talented English physicist G. Mozle found that the charge of atomic nuclei increases sequentially from element to element per unit. With this discovery, Mozle confirmed the ingenious guess of Mendeleev, who, in three places in the periodic table, moved away from the increasing sequence of atomic weights.
Thus, when compiling it, Mendeleev put 27 Co in front of 28 Ni, 52 Ti in front of 5 J, 18 Ar in front of 19 K, despite the fact that this contradicted the formulation of the periodic law, that is, the arrangement of elements in order of increasing their atomic weights.
According to the law of Mozle, the charges of the nucleated data elements corresponded to their position in the table.
In connection with the discovery of the law of Mozle, the modern formulation of the periodic law is as follows:
the properties of elements, as well as the forms and properties of their compounds are in a periodic dependence on the nuclear charge of their atoms.
Connection of the periodic law and the periodic system with the structure of atoms.
So, the main characteristic of an atom is not the atomic mass, but the magnitude of the positive charge of the nucleus. This is a more general accurate characteristic of the atom, and hence the element. All the properties of the Element and its position in the periodic system depend on the magnitude of the positive charge of the nucleus of an atom. In this way, the sequence number of a chemical element is numerically the same as the charge of the nucleus of its atom. The periodic system of the elements is a graphic representation of the periodic law and reflects the structure of the atoms of the elements.
The theory of the structure of the atom explains the periodic change in the properties of elements. An increase in the positive charge of atomic nuclei from 1 to 110 leads to a periodic repetition of the elements of the structure of the external energy level of the atoms. And since properties of elements mainly depend on the number of electrons at the external level; then they are periodically repeated. This is the physical meaning of periodic law.
As an example, consider the change in properties of the first and last elements of periods. Each period in the periodic system begins with the elements atoms, which on the external level have one s-electron (incomplete external levels) and therefore exhibit similar properties - the valence electrons easily give off, which causes their metallic character. These are alkali metals - Li, Na, K, Rb, Cs.
The period ends with elements whose atoms at the external level contain 2 (s 2) electrons (in the first period) or 8 (s 1 p 6) electrons (in all subsequent ones), that is, they have a completed external level. These are the noble gases He, Ne, Ar, Kr, Xe, which have inert properties.
Due to the similarity of the structure of the external energy level, their physical and chemical properties are similar.
In each period, with an increase in the serial number of the elements, the metallic properties gradually weaken and the nonmetallic properties increase, the period ends with an inert gas. In each period, with an increase in the serial number of the elements, the metallic properties gradually weaken and the nonmetallic properties increase, the period ends with an inert gas.
In the light of the theory of the structure of the atom, it becomes clear that the division of all elements into seven periods, made by DI Mendeleev. The number of the period corresponds to the number of energy levels of the atom, that is, the position of the elements in the periodic system is due to the structure of their atoms. Depending on which sublevel is filled with electrons, all elements are divided into four types.
1. s-elements. The s-sublevel of the outer layer is filled (s 1 - s 2). This includes the first two elements of each period.
2. p-elements. The p-sublevel of the external level is filled (p 1 - p 6) - This includes the last six elements of each period, starting with the second.
3. d-elements. The d-sublevel of the last level (d1 - d 10) is filled, and 1 or 2 electrons remain at the last (external) level. These include the elements of inserted decades (10) of large periods, starting from the 4th, located between the s-and p-elements (they are also called transition elements).
4. f-elements. The f-sublevel of the deep (third of its outside) level (f 1 -f 14) is filled, and the structure of the external electronic level remains unchanged. These are lanthanides and actinides, which are in the sixth and seventh periods.
Thus, the number of elements in the periods (2-8-18-32) corresponds to the maximum possible number of electrons at the corresponding energy levels: two at the first, eight at the second, eighteen at the third, and thirty two at the fourth. The division of groups into subgroups (main and side) is based on the difference in the filling of energy levels with electrons. The main subgroup is s - and p-elements, and a side subgroup - d-elements. Each group combines elements whose atoms have a similar structure of the external energy level. In this case, the atoms of the elements of the main subgroups contain on the external (last) levels the number of electrons equal to the group number. These are the so-called valence electrons.
The elements of the side subgroups have valence electrons of not only external, but also the penultimate (second from the outside) levels, which is the main difference in the properties of the elements of the main and side subgroups.
It follows that the group number, as a rule, indicates the number of electrons that can participate in the formation of chemical bonds. This is the physical meaning of the group number.
From the standpoint of the theory of the structure of the atom, the increase in the metallic properties of the elements in each group with increasing nuclear charge of the atom is easily explained. Comparing, for example, the level distribution of electrons in atoms 9 F (1s 2 2s 2 2p 5) and 53J (1s 2 2s 2 2p 6 3s 2 Zr 6 3d 10 4s 2 4 p 6 4 d 10 5s 2 5p 5) it can be noted that they have 7 electrons each at the external level, which indicates the similarity of properties. However, the external electrons in the iodine atom are farther from the nucleus and therefore weaker held. For this reason, iodine atoms can donate electrons or, in other words, exhibit metallic properties, which is not typical of fluorine.
So, the structure of atoms determines two regularities:
a) a change in the properties of elements horizontally - in the period from left to right, metallic and weakened non-metallic properties;
b) changing the properties of elements vertically - in the group, with the increase in the serial number, the metallic properties are enhanced and the nonmetallic ones weaken.
In this way: as the nuclear charge of atoms of chemical elements increases, the structure of their electronic shells periodically changes, which is the cause of a periodic change in their properties.
3. Structure periodic Systems D. I. Mendeleev.
The periodic system of DI Mendeleev is subdivided into seven periods — horizontal sequences of elements arranged in ascending order of the sequence number, and eight groups — sequences of elements with atoms of the same type of electronic configuration and similar chemical properties.
The first three periods are called small, the rest - large. The first period includes two elements, the second and third periods - eight each, the fourth and fifth periods - eighteen, the sixth one - thirty two, the seventh (unfinished) - twenty one elements.
Each period (excluding the first) begins with an alkali metal and ends with a noble gas.
Elements of 2 and 3 periods are called typical.
Small periods consist of one row, large - from two rows: even (upper) and odd (lower). Metals are located in even rows of large periods, and the properties of elements from left to right change little. In the odd series of large periods, the properties of elements change from left to right, as in elements of 2 and 3 periods.
In the periodic system for each element its symbol and sequence number, the name of the element and its relative atomic mass are indicated. The coordinates of the position of an element in the system are the period number and the group number.
Elements with serial numbers 58-71, called lanthanides, and elements with numbers 90-103 - actinides - are placed separately at the bottom of the table.
Groups of elements, denoted by Roman numerals, are divided into main and secondary subgroups. The main subgroups contain 5 elements (or more). Secondary subgroups include elements of periods, starting with the fourth.
The chemical properties of elements are determined by the structure of their atom, and more precisely the structure of the electron shell of atoms. Comparison of the structure of the electron shells with the position of the elements in the periodic system allows us to establish a number of important regularities:
1. The period number is equal to the total number of energy levels filled by electrons of the atoms of a given element.
2. In small periods and odd series of large periods, as the positive charge of nuclei grows, the number of electrons at the external energy level increases. This is due to the weakening of the metal and strengthening the non-metallic properties of the elements from left to right.
The group number indicates the number of electrons that can participate in the formation of chemical bonds (valence electrons).
In subgroups, with the growth of the positive charge of atomic nuclei, their metallic elements are enhanced and nonmetallic properties are weakened.
Periodicd.I.'s law Mendeleev:Properties of simple bodies, as well as the shape and properties of compoundselements are periodically dependent onthe magnitudes of the atomic weights of the elements. (The properties of electrons are in a periodic dependence on the charge of the atoms of their nuclei).
Periodic system of elements. Rows of elements, within which properties change sequentially, such as, for example, a series of eight elements from lithium to neon or from sodium to argon, Mendeleev called periods. If we write these two periods one under the other so that sodium is under lithium and argon under neon, we get the following arrangement of elements:
With this arrangement, elements similar in their properties and having the same valency fall into vertical columns, for example, lithium and sodium, beryllium and magnesium, etc.
Dividing all the elements into periods and having one period under another so that the elements similar in properties and type of compounds formed fall under each other, Mendeleev compiled a table called by him the periodic system of elements into groups and rows.
The value of the periodic systemwe.Periodic system of elements had a great influence on the subsequent development of chemistry. It was not only the first natural classification of chemical elements, which showed that they form a coherent system and are closely related to each other, but also was a powerful tool for further research.
7. Periodic changes in the properties of chemical elements. Atomic and ionic radii. Ionization energy. Affinity for the electron. Electronegativity
The dependence of atomic radii on the nuclear charge of an atom of Z is periodic in nature. Within the same period with increasing Z, a tendency to a decrease in the size of an atom appears, which is especially clearly observed in short periods
With the beginning of the development of a new electron layer, more distant from the core, i.e., upon transition to the next period, atomic radii increase (compare, for example, the radii of fluorine and sodium atoms). As a result, within the subgroup with the increase of the nuclear charge, the sizes of the atoms increase.
The loss of electron atoms leads to a decrease in its effective size, and the addition of excess electrons to an increase. Therefore, the radius of a positively charged ion (cation) is always smaller, and the radius of a negatively charged non (anion) is always greater than the radius of the corresponding electroneutral atom.
Within one subgroup, the radii of ions of the same charge increase with increasing nuclear charge. This pattern is explained by the increase in the number of electron layers and the growing distance of external electrons from the nucleus.
The most characteristic chemical property of metals is the ability of their atoms to easily release external electrons and turn into positively charged ions, and nonmetals, on the contrary, are characterized by the ability to attach electrons with the formation of negative ions. To detach an electron from an atom with its transformation into a positive ion, it is necessary to expend some energy, called the ionization energy.
The ionization energy can be determined by bombarding atoms with electrons accelerated in an electric field. The smallest field voltage at which the electron velocity becomes sufficient for the ionization of atoms is called the ionization potential of the atoms of a given element and is expressed in volts. With the expenditure of sufficient energy, two, three or more electrons can be detached from the atom. Therefore, we speak of the first ionization potential (the energy of separation from the atom of the first electron). The second potential of ionization (the energy of separation of the second electron)
As noted above, atoms can not only donate, but also attach electrons. The energy released by the attachment of an electron to a free atom is called the affinity of the atom to the electron. Electron affinity, like ionization energy, is usually expressed in electron volts. Thus, the electron affinity for the hydrogen atom is 0.75 eV, oxygen — 1.47 eV, fluorine — 3.52 eV.
The electron affinity of metal atoms is usually close to zero or negative; From this it follows that for atoms of most metals the attachment of electrons is energetically unfavorable. The affinity for an electron of non-metal atoms is always positive, and the more, the closer to a noble gas is a non-metal in the periodic system; this indicates an increase in non-metallic properties as it approaches the end of the period.
Chemical elements DI Mendeleev - the basis of modern chemistry. They belong to such scientific laws that reflect the phenomena that really exist in nature, and therefore never lose their value.
Their discovery was prepared by the entire course of the history of chemistry, but it took the genius of DI Mendeleev, his gift of scientific foresight, so that these patterns were formulated and graphically presented in the form of a table. We will use modern synonyms of the terms used by the great Russian chemist.
Prerequisites for the discovery of the Periodic Law D. I. Mendeleev
Accumulation of factual material
By the time of the opening of the Periodic Law, 63 chemical elements were known, the composition and properties of their numerous compounds were described.
Works of scientists - predecessors of D. I. Mendeleev
Berzelius classification. The eminent Swedish chemist J.Y. Berzelius divided all elements into metals and nonmetals based on differences in the properties of simple substances and compounds formed by them. He determined that basic oxides and bases correspond to metals, and acid oxides and acids to nonmetals.
But there were only two groups; they were large and included elements that were significantly different from each other. The presence of amphoteric oxides and hydroxides in some metals caused confusion. The classification was unsuccessful.
Debereiner Triad (1816). The German chemist I. V. Debereiner divided the elements by three on the basis of the similarity in the properties of the substances formed by him and so that the value that we now understand as the relative atomic mass (Am) of the middle element was equal to the arithmetic average of the two extreme ones. Triad example: Li, Na, K.
And r (Na) = (7 + 39): 2 = 23
Examples of other triads include:
The work of I. Debereiner served as confirmation of the idea that there is a definite connection between the atomic masses and the properties of the elements. But he managed to make only four triads, to classify all the elements known at that time he failed.
Spiral Shankurtua (1862). Professor of the Paris School of Higher Education A. Beguier de Chancourtis suggested arranging the elements in a spiral or cylinder forming in the order of increasing their atomic masses and indicated that in this case one can notice the similarity of the properties of the substances formed by the elements if they fall on the same vertical line of the cylinder one above the other, for example:
Octaves newlands(1865). The American chemist D. A. R. Newlands tried to arrange elements known to him in ascending order of their atomic masses and discovered a striking similarity between every eighth element, starting with any, like the structure of a musical octave consisting of eight sounds. He called his discovery the law of the octaves:
[]
However, he was unable to satisfactorily explain the pattern found, moreover, in his table there was no place not open for the elements yet, and elements that sharply differed in their properties fell into some vertical columns. The London Chemical Society met his octave law indifferently and suggested that Newlands try to arrange the elements alphabetically and identify any pattern. Table Meier (1864). German researcher L. Meyer also arranged the chemical elements in order of increasing their atomic masses: 
But in this table Meyer placed only 27 elements, that is, less than half known at that time. The location of the remaining elements: B, Al, Cu, Ag, and others - remained unclear, and the structure of the table was uncertain.
Before DI Mendeleev, about 50 attempts were made to classify chemical elements. Most scientists have tried to identify the relationship between the chemical properties of elements and their compounds and the atomic mass. But it was not possible to create a classification that includes all the chemical elements known at the time. None of the attempts led to the creation of a system that reflects the interrelation of elements and reveals the nature of their similarities and differences. The discovery of the Periodic Law and the construction of the Periodic system of chemical elements is the merit of the great Russian scientist DI Mendeleev.
In contrast to the work of predecessors, the table of the Periodic Table of Chemical Elements proposed by D.I. Mendeleev had a clear structure in the form of groups and periods (with rows), in which there was a place not only for all elements known at that time, but empty spaces were left not open. The system of D. I. Mendeleev allowed not only to predict the existence of unknown elements, but also to predict their properties, correct incorrectly determined atomic masses of already known elements.
Congress of Chemists in Karlsruhe
The third prerequisite for the opening of the Periodic Law was the decision of the International Congress of Chemists in Karlsruhe in 1860, when the atomic-molecular doctrine was finally established, the first uniform definitions of the concepts of a molecule and an atom, as well as atomic weight, which we now call relative atomic mass ( Ar). This concept as a constant characteristic of the atoms of chemical elements DI Mendeleev based his classification. He wrote: “The mass of a substance is just such a property of it, on which all other properties should depend. Therefore, it is closer or more natural to look for a relationship between the properties and similarities of elements, on the one hand, and their atomic weights, on the other. ”
The predecessors of DI Mendeleev compared only similar elements to each other, and therefore could not discover the Periodic Law. In contrast, DI Mendeleev discovered periodicity in changing the properties of chemical elements arranged in ascending order of their atomic masses, comparing all the elements known to him, including dissimilar ones.
DI Mendeleev in his discovery relied on clearly formulated starting points:
The general invariable property of atoms of all chemical elements is their atomic mass;
The properties of elements depend on their atomic masses;
The form of this dependence is periodic.
The above considerations can be called objective, that is, independent of the scientist’s personality, since they were due to the historical development of chemistry as a science.
But without the personal qualities of the great chemist, who make up the fourth, subjective prerequisite for the discovery of the Periodic Law, it would hardly have been discovered in 1869. If some other chemist had discovered it, it would probably have happened much later. The encyclopaedic knowledge, scientific intuition, the ability to generalize, the constant desire for knowledge of the unknown, the gift of scientific foresight DI Mendeleev played a significant role in the discovery of the Periodic Law.
Discovery of DI Mendeleev of the Periodic Law
The basis of his work on the classification of chemical elements DI Mendeleev put two of their main and permanent features: the value of the atomic mass and properties. He wrote out on the cards all known information about the open and studied at that time chemical elements and their compounds. Comparing this information, the scientist has compiled natural groups of elements of similar properties, the comparison of which among themselves showed that even elements of dissimilar groups have unifying features. For example, the atomic masses of fluorine and sodium, chlorine and potassium (inert gases were not yet known) are close in value, therefore, alkali metals and halogens can be put side by side, arranging chemical elements in ascending order of atomic masses. So DI Mendeleev combined the natural groups of chemical elements into a single system.
Dmitri Ivanovich Mendeleev (1834-1907)
The great Russian scientist, one of the founders of modern chemistry. The creator of the natural classification of chemical elements - the Periodic Table of Elements, which was the expression of the Periodic Law of Chemical Elements. He created a fundamental work - the textbook "Fundamentals of Chemistry", in which for the first time all inorganic chemistry is described on the basis of the Periodic Law. He is the author of the chemical theory of solutions. In his writings, he paid much attention to the development of domestic industry and the chemicalization of agriculture.
DI Mendeleev argued for the creation of chemical plants: soda, sulfuric acid, mineral fertilizers. He justified the idea of underground coal gasification and the use of oxygen in the metallurgical industry. He proposed a method of continuous processing of oil, as well as the original theory of its origin.
At the same time, he found that the properties of elements vary within their specific sets linearly (monotonously increase or decrease), and then repeat periodically, that is, after a certain number of elements, there are similar ones. The scientist identified periods in which the properties of chemical elements and the substances formed by them naturally change. Consider these changes using modern terms.
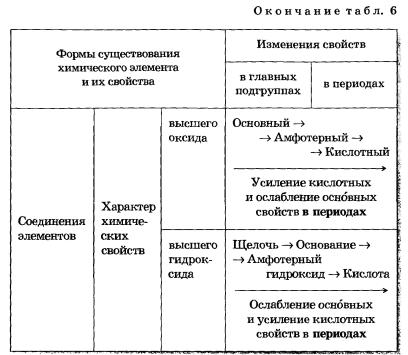
1. The metallic properties of simple substances, most pronounced in alkali metals, weaken and are replaced by non-metallic, which are most pronounced in halogens.
2. The value of the degree of oxidation of atoms of elements in higher oxides increases from +1 to +7 (+8 only for Os and Ru).
3. The value of the degree of oxidation of atoms of elements in hydrides (metal compounds with hydrogen) and in volatile hydrogen compounds increases first from +1 to +3 and then from -4 to -1.
4. The main oxides of the elements of the beginning of the period are replaced by amphoteric oxide and then acidic, whose properties are enhanced.
5. Base hydroxides through amphoteric hydroxide are replaced by more and more strong acids.
Based on these observations, DI Mendeleev formulated the Periodic Law, which, in accordance with currently accepted terminology, reads as follows:
The properties of chemical elements and the substances formed by them are periodically dependent on their relative atomic masses.
To illustrate this law, we used the considered periodicities (discreteness, discontinuity at certain intervals) only horizontally. However, the Periodic Law and the Periodic System are much richer in periodic patterns: in addition to the considered horizontal (in periods) periodicity, there is also a periodicity in the vertical (in groups) and diagonal.
Vertical periodicity is already well known to you: in groups (main subgroups) with the growth of the ordinal numbers of elements, the metallic properties of simple substances formed by them increase and the nonmetallic properties weaken; increases the basic nature of oxides and hydroxides; the strength of volatile hydrogen compounds decreases and, accordingly, their acidic properties increase.
Under the diagonal periodicity understand the repeatability of the similarity of the chemical properties of simple substances and compounds of elements located diagonally from each other in the Periodic System.
The similarity in properties between simple substances and compounds formed by chemical elements located diagonally is explained by the fact that the growth of non-metallic properties in the periods from left to right is approximately balanced by the effect of increasing the metallic properties in groups from top to bottom.
For example, lithium metal Li is similar to magnesium in everything that distinguishes it from sodium Na. Similarly, boron B is more like silicon than aluminum Al. 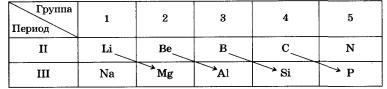
The common chemical properties of Li and Mg include their ability to easily ignite, the instability of their nitrates and carbonates, and the low solubility of fluorides, phosphates, and silicates in water.
The diagonal similarity of Be and Al is expressed in the fact that both metals react in the same way with acids and alkalis, and their oxides and hydroxides are amphoteric.
Boron and silicon form similar simple substances that are inert and refractory, and oxides and hydroxides have acidic properties. Boron, like carbon and silicon, forms volatile hydrogen compounds, according to production methods and properties similar to silicones (silanes): B2H6, B4H10, etc.
Best of all, the diagonal periodicity of the properties of non-metals is characterized by the well-known diagonal В - Si-As-Te-Аt, which conditionally divides the elements into metals and non-metals, or the diagonal C - P - Se - I.
Two diagonals: Al - Ge - Sb and Zn - In - Pb - include elements whose oxides and hydroxides have amphoteric properties.
If you combine the horizontal, vertical and diagonal periodicity, you can get a "stellar periodicity."
It is the consideration of all types of periodicity that allowed D.I. Mendeleev not only to predict, describe the properties of substances formed by not yet discovered chemical elements, but also indicate the ways of their discovery, natural sources (ores and compounds) from which the corresponding simple substances could be obtained.
Periodic law and the structure of the atom
The formulation of the law given by D. I. Mendeleev could not be exact and complete from the modern point of view, since it corresponded to the state of science at that period of time when the structure of the atom was not known. Therefore, new scientific discoveries have come into conflict with it. So, isotopes were discovered.
Isotopes - varieties of atoms of the same chemical element, having the same nuclear charge, but different mass numbers.
The sum of the numbers of protons and neutrons in the nucleus of an atom is called the mass number and is denoted by the letter A.
Obviously, the nuclei of isotopes of a single chemical element have the same number of protons, but differ in the number of neutrons contained in them. Consequently,
chemical element - is a type of atoms characterized by the same nuclear charge, that is, containing the same number of protons.
Isotopes are known for all chemical elements. In nature, most of them exist as a mixture of isotopes. The relative atomic mass of an element is equal to the average value of the relative atomic masses of all its natural isotopes, taking into account their abundance.
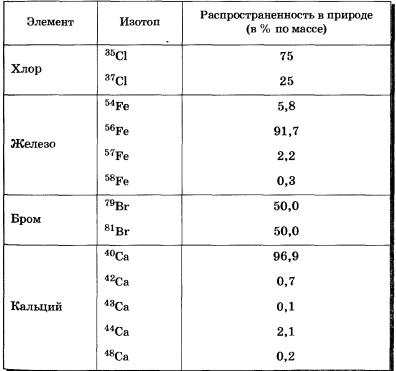
Data on isotopes of some chemical elements are given in table 5, their masses and percentage in nature (by weight) are indicated.
The periodic table below the symbols of chemical elements shows the average values of their relative atomic masses. They can be calculated by knowing the mass number of each isotope and its mass fraction in the natural mixture. So,
And r (Cl) = 35 0.75 + 37 0.25 = 35.5
Table 5 Isotopes of some chemical elements
The presence of isotopes proves that the properties of chemical elements are determined not so much by their atomic mass, as DI Mendeleev assumed, as by the charge of atomic nuclei. This explains the position in the Periodic System of four pairs of elements placed in violation of the principle of increasing relative atomic masses:
18Ag (39.948) - 19K (39.102) 27So (58.933) - 28№ (58.71) 52Te (127.60) - 531 (126.904) 90T (232.038) - 91Pa (231)
That is the genius, the manifestation of the scientific intuition of the great Russian chemist, that in these cases he preferred to arrange elements in similarity in properties, he predicted the true order of placement of chemical elements by increasing the charges of their atomic nuclei, although he did not know anything about the structure of their atoms.
For the first time, the physical meaning of the ordinal (atomic) number was revealed by the Dutchman Van-den-Bruck, who theoretically proved that the ordinal number of a chemical element is equal to the charge of the nucleus of its atom. Van-van-Bruck’s hypothesis was experimentally confirmed by the Englishman G. Mosley.
The discovery of isotopes and the van den Broek-Mosley pattern made it possible to give a different, modern definition of the Periodic Law:
The properties of chemical elements and the substances formed by them are periodically dependent on the charges of their atomic nuclei.
Periodic system of chemical elements and the structure of the atom
The table of the Periodic system of chemical elements graphically displays the Periodic law. Each number in it characterizes any feature in the structure of atoms:
a) the ordinal (atomic) number of a chemical element indicates the charge of its atomic nucleus, that is, the number of protons contained in it, and since the atom is electrically neutral, it also indicates the number of electrons around the atomic nucleus. The number of neutrons is determined by the formula
N = A - Z
where A is the mass number, Z is the sequence number of the element;
b) the period number corresponds to the number of energy levels (electronic layers) in the atoms of the elements of a given period;
c) the group number corresponds to the number of electrons at the external level for the elements of the main subgroups and the maximum number of valence electrons for the elements of the secondary subgroups.
In the light of the structure of the atom, one can explain the reasons for the changes in the properties of chemical elements and the substances formed by them. In the period with an increase in the charges of the atomic nuclei of the elements (from left to right), the metallic properties weaken, and the non-metallic ones increase due to the fact that:
a) the number of electrons increases at the outer level of the atom;
b) the number of energy levels in atoms within a period remains constant;
c) decreases the radius of the atoms.
In groups (the main subgroup), with an increase in the charges of the atomic nuclei of the elements (from top to bottom), the metallic properties increase and the nonmetallic properties weaken. This is explained by
The number of electrons at the outer level of atoms remains the same;
The number of energy levels in an atom increases;
The radius of atoms increases.
In large periods, such changes occur more slowly, since, starting with the third element, the atoms do not complete the external energy level, but the pre-external level from 8 to 18 electrons (for elements of the side subgroups), and only then the external level is filled from 2 to 8 electrons ( elements of the main subgroups).
In the "super-large" periods (the sixth and seventh, unfinished) these changes occur even more slowly, since the lanthanides and actinides do not complete the external pre-outer level, but the third outside level - from 18 to 32 electrons. Therefore, the properties of these elements will be so similar to the properties of the elements La and Ac, and also similar to each other. This is explained by the fact that the properties of chemical elements and the substances formed by them depend primarily on the structure of the external energy level of atoms, less - on the structure of the pre-outer one and almost independent of the structure of the internal levels.
The nature of each chemical element, that is, the specific, intrinsic properties of atoms, simple substances, compounds, depends primarily on the charge of the nucleus of its atoms. The charge determines the structure of the electron shell of an atom. But the magnitudes of the nuclear charges of atoms of chemical elements in the Periodic System of DI Mendeleev change monotonically - they increase from +1 for hydrogen to +110 for element No. 110, therefore this phenomenon cannot be a direct cause of a periodic change in the properties of elements.
The reason for the periodicity is a change in the structure of the outer electronic layers of atoms. Thus, for all alkali metals, the external energy level is occupied by one in-electron, therefore their properties are so similar. But they are not the same, the degree of their manifestation is different, because this single external electron is at a different distance from the nucleus of the atoms of each of the alkali metals:

The properties of chemical elements and the substances formed by them are periodically dependent on the structure of the outer electronic layers of atoms.
More complete information about the horizontal and vertical dependencies of the properties of atoms, simple substances and compounds formed by chemical elements is presented in Table 6.
Table 6 Changes in the properties of atoms, simple substances and compounds of chemical elements

The value of the Periodic Law and the Periodic Table of the Chemical Elements DI Mendeleev
DI Mendeleev wrote: “Before the periodic law, the elements were only fragmentary random phenomena of nature; there was no reason to wait for any new ones, but those that were again found were a completely unexpected novelty. The periodic regularity of the first made it possible to see the elements that were not yet open in such a distance, until which the unarmed vision did not reach until then. ”
With the discovery of the Periodic Law, chemistry ceased to be a descriptive science - it received a tool of scientific foresight. This law and its graphic display - the table of the Periodic Table of the Chemical Elements of DI Mendeleev - fulfilled all three important functions of theoretical knowledge: generalizing, explaining and prognostic. Based on them, scientists:
Systematized and generalized all information about chemical elements and the substances formed by them;
Gave a rationale to various types of periodic dependence existing in the world of chemical elements, explaining them on the basis of the structure of the atoms of the elements;
They predicted, described the properties of not yet discovered chemical elements and the substances formed by them, and also indicated the ways of their discovery.
DI Mendeleev himself had to systematize and summarize information about chemical elements when he discovered the Periodic Law, built and improved his table. Moreover, errors in the values of atomic masses and the presence of not yet open elements created additional difficulties. But the great scientist was firmly convinced of the truth of the revealed law of nature. Based on the similarity in properties and believing in the correctness of determining the location of elements in the Periodic Table, he significantly changed the atomic masses and valences accepted at that time in compounds with oxygen in ten elements and “corrected” them in ten others. He placed the eight elements in the table, contrary to the ideas of their similarity with others. For example, he excluded thallium from the natural family of alkali metals and placed it in the third group according to the highest valence it exhibits; he transferred beryllium with an incorrectly defined atomic mass (13) and valency III from the third group to the second, changing its atomic mass by 9 and the highest valence by II.
Most scientists perceived the amendments of D.I. Mendeleev as a scientific frivolity, unreasonable audacity. The periodic law and the table of chemical elements were considered as a hypothesis, that is, an assumption that needs to be checked. The scientist understood this and precisely for checking the correctness of the law discovered by him and the system of elements he described in detail the properties of the elements that were not yet open, and even the ways of their discovery, proceeding from the supposed place in the system. According to the first variant of the table, he made four predictions (gallium, germanium, hafnium, scandium), and according to the improved one, the second one - seven more (technetium, rhenium, astatine, francium, radium, actinium, protactinium).
For the period 1869-1886 three predicted elements were discovered: gallium (P.E. Lecoq de Boisbodran, France, 1875), scandium (L.F. Nilsson, Sweden, 1879) and germanium (K.Winkler, Germany, 1886) . The discovery of the first of these elements, which confirmed the correctness of the forecast of the great Russian scientist, caused only interest and surprise among his colleagues. The discovery of Germany was a true triumph of the Periodic Law. K. Winkler wrote in the article “Report on Germany”: “There is no longer any doubt that the new element is nothing but predicted by Mendeleev fifteen years before this ekacilicium. For there can hardly be given more convincing proof of the validity of the doctrine of the periodicity of elements than the embodiment of the formerly hypothetical Ekacilicia, and it is truly more than a simple confirmation of a boldly advanced theory — it represents an outstanding expansion of the chemical field of view, a powerful step. in the field of knowledge.
On the basis of the law and the table of DI Mendeleev, noble gases were predicted and discovered. And now this law serves as a guiding star for the discovery or artificial creation of new chemical elements. For example, it can be argued that the element with the number 114 will be similar to lead (Ekasvinets), and the number 118 will be a noble gas (ecradon).
The discovery of the Periodic Law and the creation of the Periodic Table of the Chemical Elements by DI Mendeleev stimulated the search for the causes of the interrelation of the elements, helped to reveal the complex structure of the atom and the development of the theory of the structure of the atom. This doctrine, in turn, made it possible to reveal the physical meaning of the Periodic Law and explain the location of the elements in the Periodic Table. It led to the discovery of atomic energy and its use for the needs of mankind.