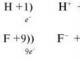Determine the type of hybridization of each carbon atom. Atomic orbitals hybridization
Covalent bond is most common in the world of organic substances, it is characterized by saturability, polarizability and orientation in space.
The covalent bond saturation is that the number of common electron pairs that an atom is capable of forming is limited. Due to this, covalent compounds have a strictly defined composition. Therefore, for example, there are molecules H 2, N 2, CH 4, but there are no molecules H 3, N 4, CH 5.
The covalent bond polarization is the ability of molecules (and individual bonds in them) to change their polarity under the influence of an external electric field - to be polarized.
As a result of polarization, non-polar molecules can become polar, and polar ones can turn into even more polar ones up to the complete rupture of individual bonds with the formation of ions:
The covalent bond orientation is due to the fact that the p-, d- and f-clouds are in a certain way oriented in space. The orientation of a covalent bond affects the shape of the molecules of substances, their sizes, interatomic distances, and the valence angle, i.e., the geometry of molecules.
A more complete picture of the shape of molecules of organic and inorganic substances can be made on the basis of the hypothesis of atomic orbitals hybridization. It was proposed by L. Pauling (USA) to explain the fact of the equivalence of all chemical bonds and their symmetrical position relative to the center of CH 4, BF 3, BeCl 2 molecules, established using physical methods of research of substances. In each case, the formation of σ-bonds from the central atom (C, B, Be) involved electrons in different states (s and p), so they could not be equivalent. The theory was unable to explain the facts, a contradiction arose, which was resolved with the help of a new hypothesis. This is one of the examples showing the development of human knowledge of the surrounding world, the possibility of ever deeper insight into the essence of phenomena.
With the hypothesis of hybridization of atomic orbitals you got acquainted in the course of organic chemistry using the example of a carbon atom. Recall this again.
When methane molecule CH4 is formed, the carbon atom from the ground state goes to the excited one:

The outer electron layer of an excited carbon atom contains one s- and three unpaired p-electrons, which form four σ-bonds with four s-electrons of hydrogen atoms. In this case, one should expect that three C – H bonds, formed by the pairing of three p-electrons of a carbon atom with three s-electrons of three hydrogen atoms (sp-σ-bond), should differ from the fourth (ss) bond by strength , length, directivity. The study of electron density in methane molecules shows that all bonds in its molecule are equivalent and directed to the vertices of the tetrahedron (Fig. 10). According to the hypothesis of hybridization of atomic orbitals, four covalent bonds of a methane molecule are formed not with the participation of “pure” s- and p-clouds of the carbon atom, but with the participation of so-called hybrid, i.e., averaged, equivalent electron clouds.

Fig. 10. Ball-stick model of methane
According to this model, the number of hybrid atomic orbitals is equal to the number of initial “pure” orbitals. Corresponding hybrid clouds are more advantageous in geometrical form than s- and p-clouds, their electron density is distributed differently, which provides a more complete overlap with s-clouds of hydrogen atoms than would be the "pure" s-and p-clouds.
In the molecule of methane and in other alkanes, as well as in all molecules of organic compounds, at the place of a single bond, carbon atoms are in the state of sp 3 hybridization, i.e., one s and three p-atomic clouds undergo a hybridization of the carbon atom and four identical hybrid sp 3-atom cloud orbitals.
As a result of overlapping of the corresponding four hybrid sp 3 clouds of a carbon atom with s clouds of four hydrogen atoms, a tetrahedral methane molecule is formed with four identical σ bonds arranged at an angle of 109 ° 28 "(Fig. 11).

Fig. eleven.
Schemes sp 3-hybridization of valence electron clouds (a) and the formation of bonds in the molecule of methane (b)
This type of hybridization of atoms and, therefore, the tetrahedral structure will also be characterized by molecules of compounds of carbon analogue - silicon: SiH 4, SiCl 4.
During the formation of water and ammonia molecules, the sp 3 hybridization of the valence atomic orbitals of oxygen and nitrogen atoms also occurs. However, if at the carbon atom all four hybrid sp 3 clouds are occupied by common electron pairs, then at the nitrogen atom one sp 3 cloud is occupied by a lone electron pair, and at the oxygen atom two sp 3 clouds are occupied by them (Fig. 12).

Fig. 12.
Forms of ammonia, water, and hydrogen fluoride molecules
The presence of lone electron pairs leads to a decrease in bond angles (Table 8) as compared with tetrahedral (109 ° 28 ").
Table 8
The relationship of the number of lone electron pairs and the bond angle in molecules

sp 3 -Hybridization is observed not only in atoms in complex substances, but also in atoms in simple substances. For example, atoms of such an allotropic modification of carbon, like diamond.
In the molecules of some boron compounds, sp 2 hybridization of the valence atomic orbitals of the boron atom takes place.

At the boron atom in the excited state, one s- and two p-orbitals participate in the hybridization, resulting in the formation of three sp 2-hybrid orbitals, the axes of the corresponding hybrid clouds are located in a plane at an angle of 120 ° to each other (Fig. 13).

Fig. 13.
Schemes 8p 2 hybridization and location of sp 2 clouds in space
Therefore, the molecules of such compounds, for example BF3, have the shape of a flat triangle (Fig. 14).

Fig. 14.
The structure of the molecule BF3
In organic compounds, as you know, sp 2 hybridization is characteristic of carbon atoms in alkenes at the double bond site, which explains the planar structure of these parts of molecules, as well as diene and arena molecules. sp 2 -Hybridization is also observed at carbon atoms and in such allotropic carbon modification as graphite.
In the molecules of some beryllium compounds, sp-hybridization of the valence orbitals of the beryllium atom is observed in the excited state.

Two hybrid clouds are oriented relative to each other at an angle of 180 ° (Fig. 15), and therefore the beryllium chloride molecule BeCl 2 has a linear shape.

Fig. 15.
Schemes of sp-hybridization and the location of sp-clouds in space
A similar type of hybridization of atomic orbitals exists at carbon atoms in alkynes — hydrocarbons of the acetylene series — at the place of the triple bond.
Such a hybridization of orbitals is characteristic of carbon atoms in one of its other allotropic modifications, carbin:
Table 9 shows the types of geometric configurations of molecules corresponding to some types of hybridization of the orbitals of the central atom A, taking into account the influence of the number of free (non-bonding) electron pairs.
Table 9
Geometric configurations of molecules corresponding to different types of hybridization of external electron orbitals of the central atom

Questions and tasks to § 7
- In the molecules of hydrogen compounds of carbon, nitrogen and oxygen, whose formulas are CH 4, NH 3 and H 2 O, the valence orbitals of the central atoms of the nonmetals are in a state of sp 3 hybridization, but the valence angles between the bonds are different - 109 ° 28 "107 ° 30" and 104 ° 27 "respectively. How can this be explained?
- Why is graphite electrically conductive and diamond not?
- What geometric shape will the molecules of two fluorides have - boron and nitrogen (BF 3 and NF 3, respectively)? Give a reasonable answer.
- The silicon fluoride molecule SiF 4 has a tetrahedral structure, and the bromine chloride molecule lCl 3 - the shape of a triangle - is planar. Why?
Hybridization is called a hypothetical mixing process of a different type, but the orbitals of a given atom that are close in energy with the appearance of the same number of new (hybrid 1) orbitals of the same energy and shape.
Hybridization of atomic orbitals occurs during the formation of covalent bonds.
Hybrid orbitals have the form of a volumetric asymmetric eight, strongly elongated in one direction from the atomic nucleus:.
Such a form causes a stronger, than in the case of pure atomic orbitals, overlap of hybrid orbitals with orbitals of (pure or hybrid) other atoms and leads to the formation of stronger covalent bonds. Therefore, the energy expended on the hybridization of atomic orbitals is more than compensated by the release of energy due to the formation of stronger covalent bonds with the participation of hybrid orbitals. The name of the hybrid orbitals and the type of hybridization are determined by the number and type of atomic orbitals participating in the hybridization, for example: sp-, sp 2 -, sp 3 -, sp 2 d- orsp 3 d 2 -hybridization.
The directionality of hybrid orbitals, and hence the geometry of the molecule, depends on the type of hybridization. In practice, the inverse problem is usually solved: first, the geometry of the molecule is established experimentally, after which the type and shape of the hybrid orbitals participating in its formation are described.
sp -Hybridization. Two hybrid sp- as a result of mutual repulsion, the orbitals are located relative to the atomic nucleus in such a way that the angle between them is 180 ° (Fig. 7).

Fig. 7. Mutual arrangement in space of two sp- single-atom hybrid orbitals: but - surfaces encompassing regions of space where the probability of an electron’s stay is 90%; b -conditional image.
As a result of this arrangement of hybrid orbitals, molecules of composition AH 2, where A is the central atom, have linear structure, that is, the covalent bonds of all three atoms are located on one straight line. For example, able sp- hybridization, the valence orbitals of the beryllium atom are located in the BeCl 2 molecule (Fig. 8). Linear configuration due to sp- the hybrid molecules of the valence orbitals of atoms also have the molecules BeH 2, Be (CH 3) 2, ZnCl 2, CO 2, HC≡N, and a number of others.
![]()
Fig. 8. Three-atom linear molecule of beryllium chloride BeCl 2 (in the gaseous state): 1 - 3r-cl orbital; 2 - two sp- hybrid orbitals of the Be atom.
s r 2 -Hybridization. Consider the hybridization of one s- and two r-orbitals. In this case, as a result of a linear combination of three orbitals, three hybrid sr 2 orbitals. They are located in the same plane at an angle of 120 ° to each other (Fig. 9). sr 2 Hybridization is characteristic of many boron compounds, which, as shown above, in the excited state has three unpaired electrons: one s- and two r-electron. When overlapping sr 2 -orbitals of the boron atom with the orbitals of other atoms form three covalent bonds, equivalent in length and energy. Molecules in which the valence orbitals of the central atom are in the state sr 2 hybridization, have a triangular configuration. The angles between covalent bonds are 120 °. Capable of sr 2 -hybridization are the valence orbitals of boron atoms in the molecules of BF 3, BC1 3, carbon atoms and nitrogen in the anions CO 3 2 -, NO 3 -.

Fig. 9. The mutual arrangement in space of three sr 2 hybrid hybrid orbitals
s r 3 -Hybridization. Substances in which the central atom contains four sr 3 orbitals resulting from a linear combination of one s- and three r-orbitals. These orbitals are located at an angle of 109˚28 ′ to each other and are directed to the vertices of the tetrahedron, in the center of which is the atomic nucleus (Fig. 10 a).
The formation of four equivalent covalent bonds due to overlap sr 3 -orbitals with orbitals of other atoms characteristic of carbon atoms and other elements of the IVA group; this causes the tetrahedral structure of the molecules (CH 4, CC 1 4, SiH 4, SiF 4, GeH 4, GeBr 4, etc.).

Fig. 10. Effect of non-bonding electron pairs on the geometry of molecules:
a - methane (there are no non-binding electron pairs);
b- ammonia (one non-binding electron pair);
at- water (two non-binding pairs).
Unbroken hybrid electronic orbit pairs lei . In all the examples considered, hybrid orbitals were “populated” by single electrons. However, there are cases when the hybrid orbital is “populated” by an electron pair. This affects the geometry of the molecules. Since the non-bonding electron pair experiences the effect of the nucleus of only its own atom, and the bonding electron pair is under the action of two atomic nuclei, the nonbonding electron pair is closer to the atomic nucleus than the bonding. As a result, a non-bonding electron pair repels binding electron pairs more strongly than they repel each other. Graphically, for clarity, a large repulsive force acting between the non-bonding and bonding electron pairs can be represented by a larger in volume electronic orbital of the bonding pair. A non-binding electron pair exists, for example, at the nitrogen atom in the ammonia molecule (Fig. 10 b). As a result of interaction with binding electron pairs, the H – N – H bond angles are reduced to 107.78 °, compared with 109.5 °, which is characteristic of a regular tetrahedron.
Binding electron pairs in a water molecule, where the oxygen atom has two nonbonding electron pairs, experience even greater repulsion. As a result, the H – O – H valent angle in the water molecule is 104.5 ° (Fig. 10 at).
If a non-binding electron pair is transformed into a binding by the donor-acceptor mechanism due to the formation of a covalent bond, then the repulsive forces between this bond and other covalent bonds in the molecule are aligned; align and angles between these connections. This occurs, for example, during the formation of the ammonium cation:
Participation in hybridization d -orbitals. If the energy is atomic d- orbitals are not very different from energies s- and r-orbitals, they can participate in hybridization. The most common type of hybridization involving d- orbitals is sr 3 d 2 - hybridization, which results in six hybrid orbitals of equal form and energy (Fig. 11 but), located at an angle of 90 ° to each other and directed to the vertices of the octahedron, in the center of which is the atomic nucleus. Octahedron (Fig. 11 b) – is a regular octahedron: all edges in it are of equal length, all faces are regular triangles.

Fig. eleven. sr 3 d 2 - Hybridization
Less common sr 3 d- hybridization with the formation of five hybrid orbitals (Fig. 12 but) directed to the vertices of the trigonal bipyramid (Fig. 12 b). The trigonal bipyramid is formed by the joining of two isosceles pyramids with a common base - a regular triangle. Bold strokes in fig. 12 b equal edges are shown. Geometrically and energetically sr 3 d- hybrid orbitals are unequal: three “equatorial” orbitals are directed to the vertices of a regular triangle, and two “axial” - up and down perpendicular to the plane of this triangle (Fig. 12 at). The angles between the "equatorial" orbitals are 120 °, as in sr 2 - hybridization. The angle between the "axial" and any of the "equatorial" orbitals is 90 °. Accordingly, covalent bonds that are formed with the participation of "equatorial" orbitals differ in length and energy from bonds in the formation of which "axial" orbitals are involved. For example, in the PC1 5 molecule, the “axial” bonds are 214 pm long, and the “equatorial” bonds are 202 pm.

Fig. 12. sr 3 d- Hybridization
Thus, considering covalent bonds as a result of overlapping atomic orbitals, one can explain the geometry of the molecules and ions that arise in this case, which depends on the number and type of atomic orbitals involved in the formation of bonds. The concept of hybridization of atomic orbitals, it is necessary to understand that hybridization is a conditional technique that allows you to visually explain the geometry of a molecule through a combination of AO.
Hybridization of orbitals and the structure of the molecules of ethane, ethylene and acetylene
Dzenis A.V.,
chemistry teacher
GBOU "School 109"
JV in FNCC DGOI
them. D. Rogacheva

"When will we know the nature closer
chemical energy very kind
atomic motion ... then teaching
about chemical structure will fall like
former chemical theories fell.
But, like most of these
theories it won't fall in order
to disappear and in order to
enter in a modified form in the range of new and wider views ... "
A.M.Butlerov
Having developed the theory of the chemical structure of organic compounds and confirming its correctness by the synthesis of new compounds, AMButlerov argued that it should and will develop.
These words were prophetic. Today we have to get acquainted with these "New views" (according to A.M. Butlerov) - electronic representations in organic chemistry .
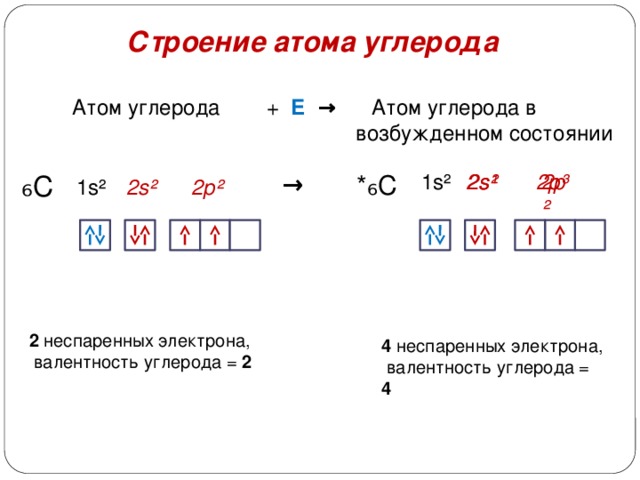
The structure of the carbon atom
Carbon atom
+ E → Carbon atom in
excited state
2 unpaired electron
carbon valence = 2
From the courses of physics and chemistry you already know that at the beginning of the 20th century it was proved that an atom forms a complex system, which consists of a nucleus and electrons.
- What is the physical meaning of the ordinal number of an element? Period numbers? Group numbers?
4 unpaired electron
carbon valence = 4

"hybridization" -
mixing and leveling
electronic clouds .
sp 3 -hybridization carbon atom
AND
structure of methane and ethane molecules

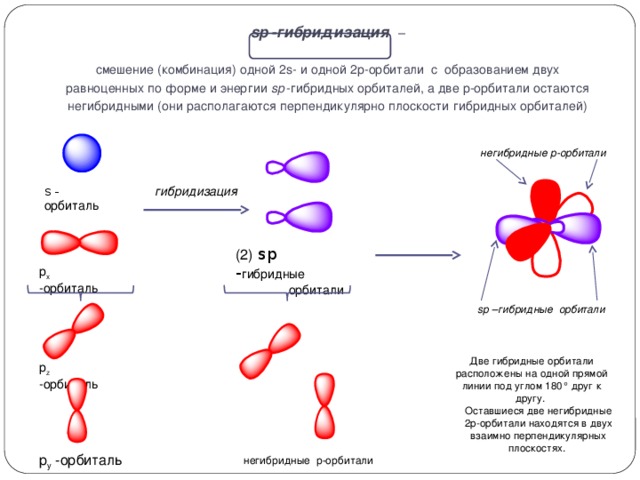
sp 3 -hybridization - mixing (combination) of one 2s- and three 2p-orbitals, with the formation of four equal in form and energy sp 3-hybrid orbitals.
s - orbital
hybridization
p z -orbital
The axes of the sp 3 hybrid orbitals are directed to the vertices of a regular tetrahedron. The tetrahedral angle between them is 109 ° 28 ", which corresponds to the lowest repulsion energy of electrons
p x orbital
(4) sp 3 - hybrid
orbitals
p y -orbital

Models with sp 3 methane (CH 4 )
Scale model
Ballpoint model

sp 3 -hybridization
Ethane Molecular Structure
S₂H ₆ - ethane
; angle α = 109˚28́;
c-C bond length = 0.154 nm.
σ-bond
along the axis connecting the nuclei of the atoms to be bonded (i.e. axially overlapping AO).

sp 2 -hybridization
carbon atom
AND
ethylene molecule structure

sp 2 -hybridization - mixing (combination) of one 2s- and two 2p-orbitals with the formation of three equal in form and energy sp 2 hybrid orbitals; one p orbital remains non-hybrid
(it is located perpendicular to the plane of hybrid orbitals)
non-hybrid p-orbital
s - orbital
hybridization
p z -orbital
sp 2 –Hybrid
orbitals
(3) sp 2 - hybrid
orbitals
p x orbital
Location
sp 2 hybrid orbitals in space, as well as non-hybrid p orbitals
non-hybrid p-orbital
p y -orbital

Models with sp 2 carbon atom by example ethylene (C 2 H 4 )
Between carbon atoms 1 - and 1 -connection
π-link occurs when lateral overlapping non-hybrid r - AO outside the direct connecting nucleus of atoms.
π-bond is weaker than σ-bond due to less complete overlap r -Aa.

sp -hybridization carbon atom
AND
acetylene molecule structure
sp -hybridization - mixing (combination) of one 2s- and one 2p-orbitals with the formation of two equal in form and energy sp - hybrid orbitals, and two p-orbitals remain non-hybrid (they are perpendicular to the plane of hybrid orbitals)
non-hybrid p-orbitals
s - orbital
hybridization
(2) sp - hybrid
orbitals
p x orbital
sp –bridge orbitals
Two hybrid orbitals are located on the same straight line at an angle of 180 ° to each other.
The remaining two non-hybrid 2p orbitals are in two mutually perpendicular planes.
p z -orbital
non-hybrid p-orbitals
p y -orbital
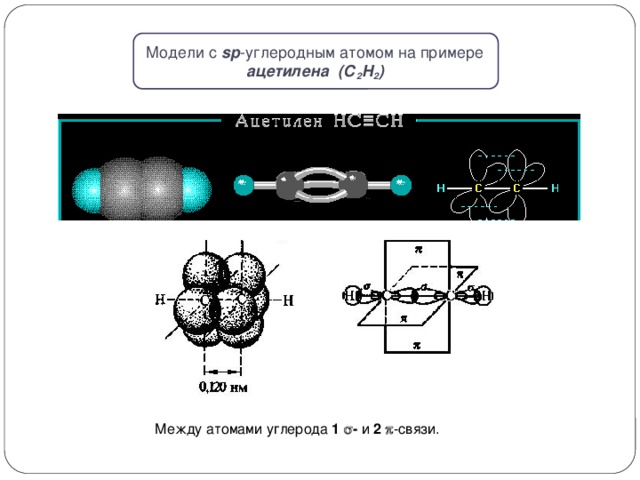
Models with sp carbon atom by example acetylene (C 2 H 2 )
Between carbon atoms 1 - and 2 -connection.
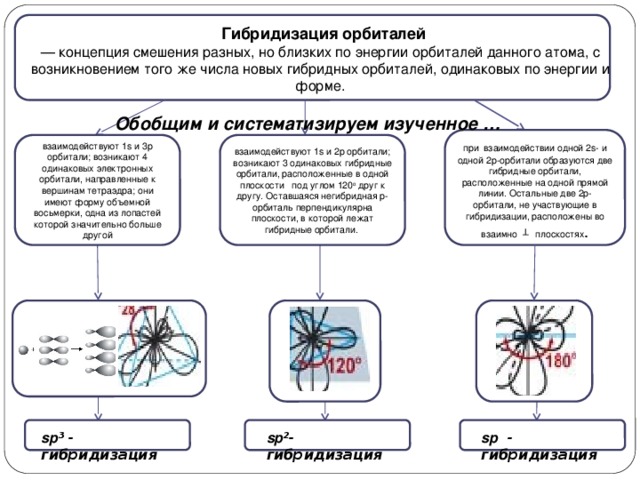
Hybridization of orbitals
The concept of mixing different but close in energy orbitals of a given atom, with the emergence of the same number of new hybrid orbitals of the same energy and shape.
Summarize and systematize the studied ...
during the interaction of one 2s and one 2p orbitals, two hybrid orbitals are formed, located on the same straight line. The remaining two 2p orbitals not participating in the hybridization are located in mutually planes.
1s and 3p orbitals interact; 4 identical electron orbitals directed toward the vertices of the tetrahedron arise; they have the shape of a volumetric eight, one of the blades of which is significantly larger than the other
1s and 2p orbitals interact; 3 identical hybrid orbitals arise in the same plane at an angle of 120 degrees to each other. The remaining non-hybrid p-orbital is perpendicular to the plane in which the hybrid orbitals lie.
sp ³ - hybridization
sp ²- hybridization
sp - hybridization

The structure of the molecules of ethane, ethylene and acetylene
Type of hybridization of carbon atoms
sp -hybridization
sp 3 -hybridization
sp 2 -hybridization
WITH ₂ H ₂ - ethin
(acetylene)
WITH 2 H 6
ethane
С₂Н₄
ethylene
Only σ-bonds between carbon atoms
σ –
and
2 π-links
σ –
and 1 π-bond
Only σ-bonds between carbon atoms
σ - bond formed when overlapping AO along the axis connecting the nuclei of atoms bound
π-link occurs when lateral overlapping non-hybrid r - AO outside the direct connecting nucleus of atoms. π-bond is weaker than σ-bond due to less complete overlap r -Aa.

thank
attention

Information sources
http://www.chemistry.ssu.samara.ru/chem1/index1.htm
http://ido.tsu.ru/schools/chem/data/res/org/uchpos/text/2_3_3.html