Molecules are made up of different kinds of atoms. The structure of matter - molecules
Quantum(from him. Quant- "quantum", from lat. quantum- "how much") - an indivisible portion of any elementary particle or quantity in physics (for example, the amount (portion) of electromagnetic radiation, which in a single act is capable of emitting or absorbing or other quantum system; elementary particle, the same as a photon). The concept is based on the concept of quantum mechanics that some physical quantities can only take certain values (they say that a physical quantity quantized).
In some important special cases, this value or the step of its change can only be integer multiples of some fundamental value - and the latter is called quantum... For example, the energy of monochromatic electromagnetic radiation of angular frequency ω can take on the values (N + 1/2) ℏω, where ℏ is the reduced Planck's constant, and N is an integer. In this case, ℏω has the meaning of the energy of a radiation quantum (in other words, a photon), and N is the meaning of the number of these quanta (photons). It is in this sense that the term quantum was first introduced by Max Planck in his classic work of 1900, the first work on quantum theory that laid its foundation.
Around the idea of quantization, since the early 1900s, a completely new physical concept has developed, usually called quantum physics (for example, the amount (portion) of electromagnetic radiation that, in a single act, is capable of emitting or absorbing, or another quantum system; an elementary particle, the same as photon).
Nowadays the adjective "quantum" is used in the name of a number of areas of physics ( quantum mechanics, quantum field theory, quantum optics, etc.). The term quantization is widely used, meaning the construction of a quantum theory of a certain system or the transition from its classical description to a quantum one. The same term is used to denote a situation in which a physical quantity can only take discrete values - for example, they say that the energy of an electron in an atom is "quantized". The very same term "quantum" currently has a rather limited application in physics. Sometimes it is used to denote particles or quasiparticles corresponding to bosonic interaction fields (photon - quantum electromagnetic field, phonon - quantum of the field of sound waves in a crystal, graviton - hypothetical quantum of the gravitational field, etc.), such particles are also referred to as "quanta of excitation" or simply "excitations" of the corresponding fields.
In addition, according to tradition, the "quantum of action" is sometimes called the Planck constant. In the modern sense, this name may have the meaning that Planck's constant is a natural quantum unit for measuring action and other physical quantities of the same dimension (for example, angular momentum).
Quark is a fundamental particle in the Standard Model with an electric charge that is a multiple of e/ 3, and not observed in a free state, but included in hadrons (strongly interacting particles such as protons and neutrons). Quarks are structureless, pointlike particles; this has been verified up to a scale of about 5 · 10 −18 m, which is about 20 thousand times smaller than the size of a proton.
At present, 6 different "sorts" (more often they say - "flavors") of quarks are known, the properties of which are given in the table. In addition, for the gauge description of the strong interaction, it is postulated that quarks also have an additional internal characteristic called "color". Each quark corresponds to an antiquark with opposite quantum numbers.
The hypothesis that hadrons are built from specific subunits was first put forward by M. Gell-Mann and, independently of him, J. Zweig in 1964.
Nuclones(from lat. nucleus- nucleus) - particles from which atomic nuclei are built. Nucleons are represented by protons and neutrons.
From the point of view of electromagnetic interaction, the proton and the neutron are different particles, since the proton is electrically charged, and the neutron is not. However, from the point of view of the strong interaction, which is decisive on the scale of atomic nuclei, these particles are indistinguishable, therefore the term "nucleon" was introduced, and the proton and neutron began to be considered as two different states of the nucleon, differing in the projection of the isotopic spin. The closeness of the properties of isospin states of a nucleon is one of the manifestations of isotopic invariance.
Atom is smallest particle chemical element keeping all of it Chemical properties... An atom consists of a nucleus, which has a positive electrical charge, and negatively charged electrons. The charge of the nucleus of any chemical element is equal to the product of Z by e, where Z is the ordinal number of the given element in the periodic table of chemical elements, and e is the value of the elementary electric charge.
Electron- the smallest particle of a substance with a negative electric charge e = 1.6 · 10 -19 coulomb, taken as an elementary electric charge. The electrons, rotating around the nucleus, are located on the electron shells K, L, M, etc. K is the shell closest to the nucleus. The size of an atom is determined by its size electronic shell... An atom can lose electrons and become a positive ion, or attach electrons and become a negative ion. The charge of an ion determines the number of lost or attached electrons. The process of converting a neutral atom into a charged ion is called ionization.
Atomic nucleus(the central part of the atom) consists of elementary nuclear particles - protons and neutrons. The radius of the nucleus is about a hundred thousand times smaller than the radius of the atom. The density of the atomic nucleus is extremely high. Protons- stable elementary particles with a single positive electric charge and a mass 1836 times greater than the mass of an electron. The proton is the nucleus of the lightest element, hydrogen. The number of protons in the nucleus is Z. Neutron- a neutral (not having an electric charge) elementary particle with a mass very close to the mass of a proton. Since the mass of the nucleus is the sum of the masses of protons and neutrons, the number of neutrons in the nucleus of an atom is equal to A - Z, where A is the mass number of a given isotope (see. Periodic system chemical elements). The proton and neutron that make up the nucleus are called nucleons. In the nucleus, nucleons are bound by special nuclear forces.
The atomic nucleus contains a huge amount of energy that is released during nuclear reactions. Nuclear reactions occur when atomic nuclei interact with elementary particles or with the nuclei of other elements. As a result of nuclear reactions, new nuclei are formed. For example, a neutron can transform into a proton. In this case, a beta particle, i.e., an electron, is ejected from the nucleus.
The transition in the nucleus of a proton to a neutron can be carried out in two ways: either a particle with a mass equal to the mass of an electron, but with a positive charge, called a positron (positron decay), is emitted from the nucleus, or the nucleus captures one of the electrons from the nearest K-shell (K - capture).
Sometimes the formed nucleus has an excess of energy (it is in an excited state) and, passing into a normal state, releases excess energy in the form of electromagnetic radiation with a very short wavelength - gamma radiation. The energy released during nuclear reactions is practically used in various industries.
A molecule (French molecule, from Latin moles - mass) is the smallest particle of a substance capable of independent existence, possessing its chemical properties.
The study of the structure and properties of molecules has acquired exceptional interest for understanding the submicroscopic structure of cells and tissues, as well as the mechanism of biological processes at the molecular level. The great advances in the study of the structure of M. and, in particular, M. of such biopolymers as proteins and nucleic acids have shown that the most important functions of these substances in organisms are carried out at the level of individual molecules and therefore must be studied as molecular phenomena. It has been established, for example, that such functions of proteins as enzymatic, structural, contractile, immune, transport (reversible binding and transfer of vital substances) are played out at the molecular level and are directly determined by the structure and properties of M. of these substances. Heredity and variability of organisms are associated with the special structure and properties of M. nucleic acids, in which all the genetic information necessary for the synthesis of body proteins is recorded. Small deviations in the structure or composition of the molecules of a number of biologically important substances or changes in the molecular mechanism of some metabolic processes cause a number of diseases (for example, sickle cell anemia, hereditary galactosemia, diabetes mellitus, etc.), called molecular diseases.
The molecule of each substance consists of a certain number of atoms (see) of one chemical element (simple substance) or various elements ( complex substance), united by chemical (valence) bonds. The composition of M. is expressed by a chemical formula, in which the signs of the elements indicate the type of atoms that form the M., and the numbers on the bottom right indicate how many atoms of each element are included in M. Thus, from chemical formula glucose СН12Ое it follows that M. glucose consists of 6 carbon atoms, 12 hydrogen atoms and 6 oxygen atoms. The molecules of inert gases and vapors of some metals are monoatomic. These are the simplest M. The most complex are M. of proteins (see), nucleic acids (see) and other biopolymers, consisting of many thousands of atoms.
All substances are made up of tiny particles - atoms. Atoms combine to form molecules, the largest of which are complex, consisting of thousands of atoms.
Even the ancient Greeks knew that everything that exists is made up of particles. Around 420 BC NS. philosopher Democritus supported the hypothesis that matter consists of tiny, indivisible particles. In Greek, atomos means "indivisible", so these particles were called atoms.
Other philosophers took a different point of view, and in the 4th century BC. NS. Aristotle supported the opinion that matter consists of various combinations of the so-called four elements - earth, air, fire and water. This idea became widespread and formed the basis of alchemy - a primitive form of chemistry that dominated science until the 17th century.
One of the main tasks of alchemy was the creation of the "elixir of life" - a drug that would allow a person to live forever. Another was to create wealth by converting common metals into gold. Many alchemists have claimed to have solved these problems, but none of them ever achieved real success.
Revolution in science
Some scientists continued to hold the view that matter is composed of atoms, but only in early XIX century, experimental data were obtained to support this theory. The English chemist and writer John Dalton experimented with gases and studied the ways of their combination. So, he found that oxygen and hydrogen, forming water, always combine in the same proportions by mass. Other scientists have also come across similar data, but it was Dalton who first realized their significance. He concluded that substances are composed of atoms, and that all atoms of a simple substance have the same mass. When connecting simple substances the numbers of connecting atoms are in a certain constant proportion. Dalton's atomistics explained why substances combine in a constant mass proportion, and also served as the basis for a detailed study of matter. Substances are made of atoms, but what are atoms made of? The first clues to this mystery appeared in the late 19th century, when researchers were studying the passage of electricity through discharge tubes containing rarefied air. Sometimes the walls of the tube emitted green light when a high voltage was applied to two metal plates - electrodes. The glow occurred when invisible rays from a negative electrode, or cathode, hit the walls of the tube.
In the 1890s, the English physicist J. Thomson proved that these cathode rays (as they were then called) are nothing more than streams of negatively charged particles. It was assumed that these particles emanate from atoms, although their location within the atoms remained unclear. Thomson suggested that the atom might be like a Christmas pudding, in which a large but lightweight positively charged sphere is littered with numerous negatively charged particles (electrons). However, various experiments on the study of the structure of the atom have proved that this is an absolutely erroneous theory.
Atom structure
In 1911, Ernest Rutherford, a New Zealand-born British physicist who worked with Thomson, proposed the structure of the atom that actually explains his behavior during experiments. Rutherford suggested that the center (or nucleus) of an atom has a positive charge and a relatively large mass, while extremely light and negatively charged electrons revolve around the nucleus.
However, Rutherford did not realize that usually in the nucleus of an atom there are both positively charged and neutral particles. The existence of positively charged particles was recognized in 1920, and they were named protons. In 1932, the English physicist James Chadwick discovered uncharged particles and called them neutrons. As a result, the picture of the structure of the atom was completed and since then it has been the basis of our understanding of matter.
The elements
Any substance in which all atoms have the same number of protons is called an element. The number of protons in each atom is atomic number element. There are 92 natural elements, their atoms have from 1 to 92 protons. In addition, some other items with more a large number protons in an atom can be produced using a device called a particle accelerator. Natural elements include iron, mercury and hydrogen.
In many substances, atoms are grouped into groups called molecules. So, hydrogen gas consists of molecules, each of which contains two hydrogen atoms. Often, however, the molecules of a substance are composed of atoms of more than one element. Such substances are called compounds. For example, water is a compound where each molecule has two hydrogen atoms and one oxygen atom. Many molecules contain much large quantity atoms. Some protein molecules are complex compounds of several thousand atoms. Some natural elements are found only in compounds. So, sodium is a metal that combines so easily with other substances that it cannot be detected in its pure form. It is widely known in combination with chlorine in the form of sodium chloride - table salt.
The atoms in molecules bind in different ways, and they share or exchange electrons. Two simple types chemical bond are covalent and ionic.
A covalent bond occurs when atoms share electrons. So, a hydrogen gas molecule consists of two hydrogen atoms linked covalent bond... A single electron of each hydrogen atom revolves around the nuclei of both atoms, binding them together.
In the case of ionic bonding, one atom transfers electrons to another atom. The result is an electrical force that binds the atoms together. Typically, the number of positively charged protons and negatively charged electrons in an atom is the same. Their positive and negative charges cancel each other out, and therefore the atom has no common charge. However, an excess of positive charge is created in the atom donating electrons, and the atom donating electrons acquires an overall negative charge. These charged atoms are called ions. Ions of opposite charges are attracted to each other, and it is this electrical attraction that holds the atoms together when ionically bonded. For example, a salt molecule is formed by ionic bonding when a sodium atom transfers an electron to a chlorine atom.
All atoms of one substance have the same number of protons, but a different number of neutrons. So, in carbon, the nucleus of most atoms contains six neutrons, but about every hundredth of them there are seven neutrons. These different types of atoms of the same element are called isotopes. All isotopes of a given element have the same chemical properties - they all combine with other substances and form the same chemical compounds... But the individual physical properties of isotopes differ - for example, they have different freezing or boiling points.
Speaking about a specific isotope of an element, scientists call its mass number. For example, carbon-12 is a common natural isotope of carbon. Its atom contains six protons and six neutrons. A rarer natural isotope with an extra neutron in the nucleus of each atom is called carbon-13.
Atomic weight
A proton and a neutron have almost the same mass, which is more than 1800 times the mass of an electron. Therefore, when it comes to the mass of an atom, as a rule, it will not be a mistake to refer to its mass number.
The atomic weight of an element, or its relative atomic mass, is usually the average mass of a mixture of naturally occurring isotopes. The molecular weight of a substance, or its relative molecular weight, is the sum of the atomic weights of all atoms in one molecule of a given substance.
Polysyllabic atom
Since then, scientists who have experimented with accelerators have discovered hundreds of other kinds of particles in atoms. But fortunately, simple model atom is sufficient to explain most of the properties of matter.
The structure of matter is one of the most common topics of debate among ancient philosophers. Since ancient times, people have made assumptions about how the matter around us is arranged, from which all objects are made. The point of view was very widespread that matter consists of fire, water, air or earth - 4 elements.
Democritus' theory of the structure of matter
Among others, there was the point of view of the ancient Greek scientist Democritus that matter consists of the smallest indivisible particles. These particles were called atoms, since the atom from the ancient Greek is translated as "indivisible". This assumption of Democritus did not attract attention for a long time, and at some times was considered blasphemy at all.
Only in the 18th century with the development of physics and chemistry, scientists were able to confirm and develop the ideas of Democritus. But the simplest representative of this or that type of metry was no longer an atom, but a molecule. But the molecule, in turn, consists of atoms.
So, for example, the water molecule H2O is the smallest representative of such a substance as water. A water molecule consists of two hydrogen atoms and one oxygen atom. By themselves, hydrogen and oxygen do not have the properties of water. On the contrary, water only becomes water when such a bond is formed.
So, matter is made up of molecules. But why don't we notice it? The answer is simple: the molecules are so small that they are simply invisible to the human eye. Only through electron microscopes can individual molecules be seen.
Which is smaller than molecules?
Molecules, in turn, as we found out, are made up of atoms. However, unlike the times of Democritus, atoms are no longer considered indivisible (which, however, did not prevent the name from being preserved). At the beginning of the 20th century, scientists managed to "cut" the atom and study the internal structure of the atom.
It turned out that an atom consists of a nucleus and an electron rotating around the nucleus. Later it turned out that the nucleus, in turn, consists of a proton and a neutron. Physics of the XXI century goes further and tries to figure out what protons, neutrons and electrons are made of. And the results that modern scientists are achieving would certainly delight Democritus.
The role of the Hadron Collider in the study of the structure of matter
So, experiments are in full swing at the Large Hadron Collider - a huge structure built underground on the border between France and Switzerland. The Large Hadron Collider is a 30-kilometer-long closed tube through which protons are accelerated. Having accelerated to almost the speed of light, the protons collide.
The force of the impact is so great that the protons are "broken" into pieces. It is assumed that in this way it is possible to study the internal structure of hadrons (the so-called proton, neutron or electron). It is obvious that the further a person goes in studying internal structure substances, the more difficulties he faces.
It is also noteworthy that the smaller the size of the desired particle, the more massive the structure for study needs to be built. Irony, however ... It is possible that the indivisible particle that Demkorit imagined for himself does not exist at all and the particles can be divided ad infinitum. Research in this area is one of the most rapidly developing topics in modern physics.
According to modern concepts:
Atom Is an electrically neutral particle consisting of a positively charged nucleus and negatively charged electrons.
It is wrong to say that "an atom is the smallest particle of a chemical element that retains all its chemical properties", because. chemical element - this is a kind of particles (atoms, ions, nuclei) with a certain charge of the nucleus; therefore the element does not consist of atoms!
In addition, chemical properties are the energy and speed of a chemical reaction, and they depend not only on the composition of the reacting particle, but also on its energy state, geometric shape, etc., therefore, not atoms (and molecules) have chemical properties, but their aggregates are chemicals.
Molecule Is the smallest electrically neutral set of atoms that form a certain structure through chemical bonds, which determines the composition of a substance.
According to modern concepts, substances in a gaseous and vapor state consist of molecules. In the solid state, only substances are made of molecules, the crystal lattice of which has molecular structure(most organic substances; non-metals, except for boron, silicon, allotropic modifications of carbon; carbon dioxide CO 2; water H 2 O).
The majority of solid inorganic substances do not have a molecular structure: their lattice does not consist of molecules, but of other particles (ions, atoms); they exist in the form of macro-bodies (NaCl crystal, quartz druse, a piece of iron, etc.). Non-molecular substances include salts, metal oxides, diamond, silicon, metals, etc.
The chemical bond between molecules in substances with a molecular structure is less strong than between atoms in a molecule, therefore, their melting and boiling points are relatively low. In substances with a non-molecular structure, the chemical bond between particles is very strong, so their melting and boiling points are high.
1.3.2. The masses of atoms and molecules. Moth
The masses of atoms and molecules are extremely small, so a special unit of measurement is used for them - atomic mass unit (abbreviated designation "a. e. m."):
1 a. unit m = 1.66 · 10 –27 kg.
For example, the absolute mass of an aluminum atom:
m o (Al) = 4.482 · 10 –26 kg = 27 amu. eat.
More often use dimensionless magnitudes- relative atomic and molecular weights.
Relative atomic mass A r - a number showing how many times the mass of a given atom is more than 1/12 of the mass of a carbon atom 12 C.
For example:
A r (Al) = = 27.
Relative molecular weight M r - a number showing how many times the mass of a given molecule is more than 1/12 of the mass of a carbon atom 12 C.
For example:
M r (SO 2) = 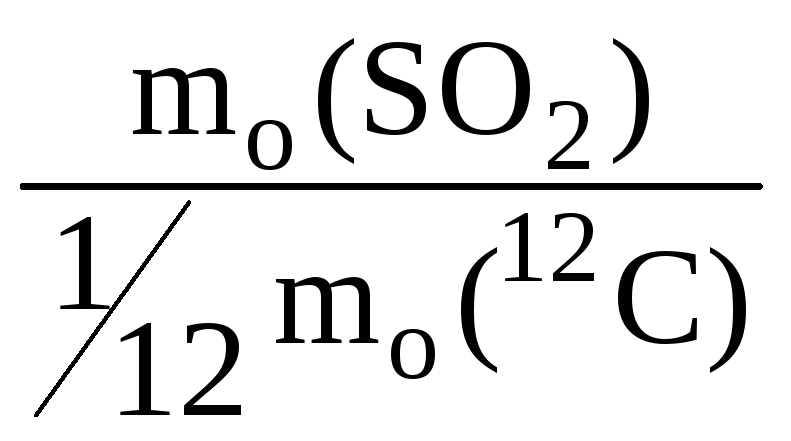 = 64.
= 64.
Along with the units of mass and volume, in chemistry they also use a unit of the amount of a substance called a mole (abbreviated designation - "mole").
Moth Is the amount of a substance containing the same number of structural units (atoms, molecules, ions, nuclei, electrons, radicals) as there are atoms in 0.012 kg (12 g) of carbon 12 C.
One mole of any substance contains Avogadro's number structural units, namely
N A = 6.02 · 10 23 mol –1.
A mole of a substance has a certain mass (molar mass) and a certain volume (molar volume).
Molar (molar) mass M Is the mass of 1 mol of a substance, expressed in mass units:
M (Al) = 27 g / mol; M (H 2 SO 4) = 98 g / mol.
Molar (molar) volume V m - the volume of 1 mol of a substance, expressed in units of volume:
V m (CO 2) = 22.4 L / mol (n.a.) 1; V m (H 2 O) = 18 ml / mol.
Example 1.1 ... During the Vietnam War (1962–1971), American troops used defoliants extensively to combat guerrillas. The "agent orange" defoliant (orange reagent) causes an accelerated fall of the leaves of trees. In total, 57 thousand tons of this preparation was sprayed over the jungle, which contained up to 170 kg of dioxin as an impurity. Now this defoliant is known under the name 2,4-D (2,4-dichlorophenoacetic acid). Calculate the mass of one defoliant molecule (molecular formula C 8 H 6 O 3 Cl 2): a) in grams; b) in atomic mass units.
Solution:
a). To calculate the mass of a 2,4-dichlorophenoacetic acid molecule, you need to know its molar mass:
M (C 8 H 6 O 3 Cl 2) = 8 12 + 6 1 + 3 16 + 2 35.5 = 221 (g / mol).
We calculate the amount of substance according to the following formulas:
ν = m / M; ν = N / N A,
where m is the mass, M is the molar mass, N is the number of atoms or molecules, N A = 6.02 · 10 23 mol –1 is Avogadro's constant.
By combining these formulas, you can express the mass in terms of the number of molecules:
m = ν M = 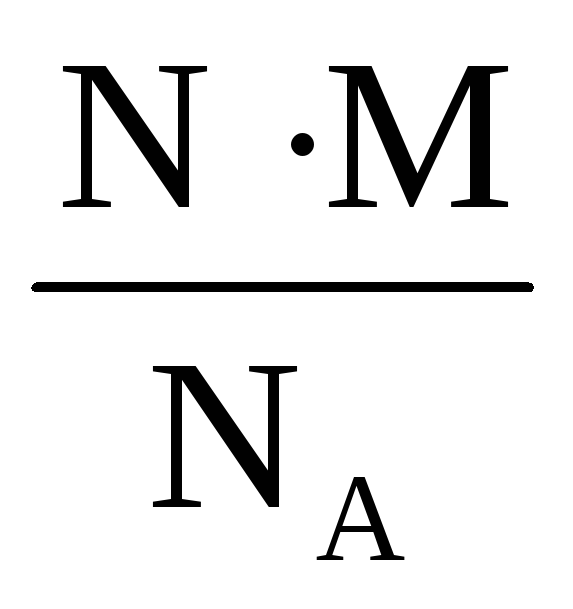 .
.
Substituting into the resulting formula N = 1, M = 221 g / mol, N A, we find:
m (C 8 H 6 O 3 Cl 2) = 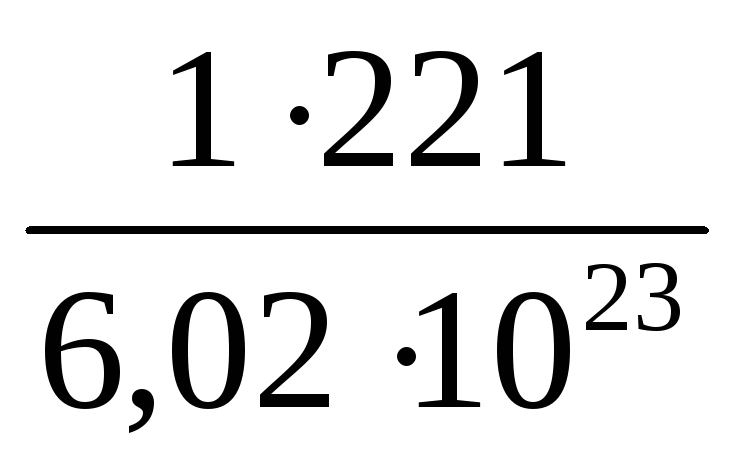 = 36.7 · 10 –23 (g).
= 36.7 · 10 –23 (g).
b). The absolute mass of a molecule is equal to the relative molecular weight multiplied by 1 amu. eat.
m (C 8 H 6 O 3 Cl 2) = 1 a. e.m.M r (C 8 H 6 O 3 Cl 2)
The relative molecular weight is numerically equal to the molar weight:
M r (C 8 H 6 O 3 Cl 2) = 221;
m (C 8 H 6 O 3 Cl 2) = 1 a. units 221 = 221 units eat.
Example 1.2. How many molecules are there in 1 liter of water?
Solution. 1. Weight 1 l water can be calculated using the density value (the density of water at 4С is equal to 1 g / cm 3):
m (H 2 O) = V (H 2 O) ρ (H 2 O);
V (H 2 O) = 1 l = 1 dm 3 = 1000 cm 3;
m (H 2 O) = 1000 cm 3 1 g / cm 3 = 1000 g.
2. Further reasoning can be carried out in two ways.
Method 1: by the amount of substance.
Using the formulas ν = m / M and ν = N / N A, we find:
ν (H 2 O) = m (H 2 O) / M (H 2 O); ν (H 2 O) = 1000 g / 18 g / mol = 55.6 mol.
N (H 2 O) = ν (H 2 O) N A; N (H 2 O) = 55.6 mol · 6.02 · 10 23 mol –1 = 334.7 · 10 23 = 3.35 · 10 25.
Method 2: using proportions.
18 g (1 mol) of H 2 O contain 6.02 · 10 23 molecules;
1000 g of H 2 O contain N molecules.
N (H 2 O) = 1000 6.02 10 23/18 = 3.35 10 25.
Example 1.3. Calculate the molar volume of aluminum if its density is 2.7 g / cm 3.
Solution. To calculate the molar volume through the density of a substance, you need to know its molar mass:
ρ (Al) = 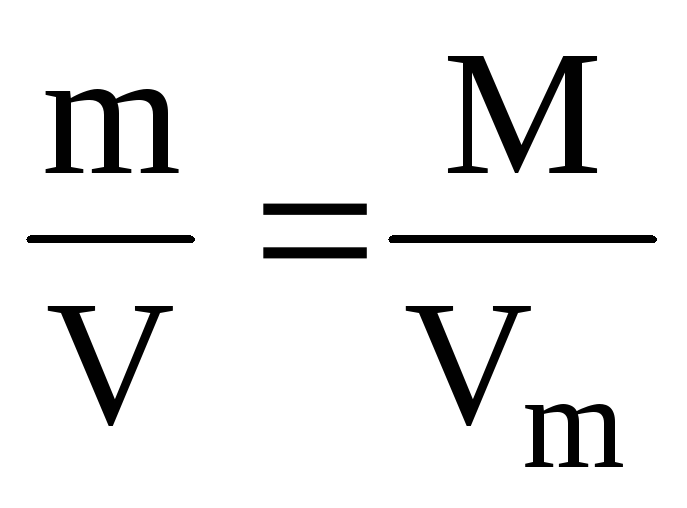 ; V m (Al) =
; V m (Al) = 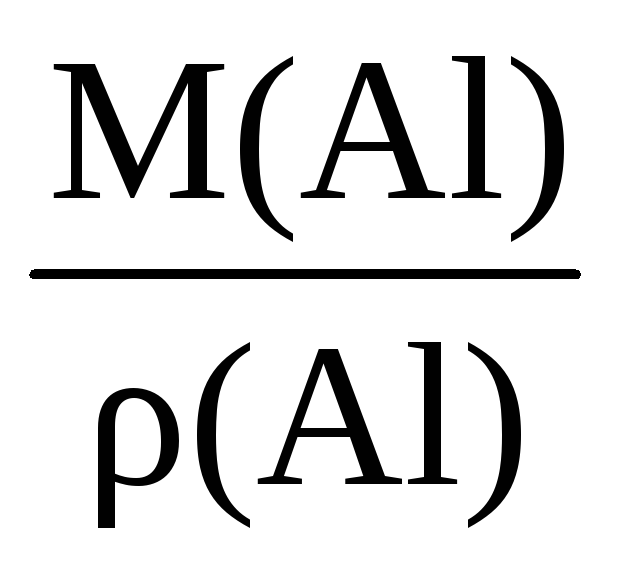 .
.
V m (Al) = 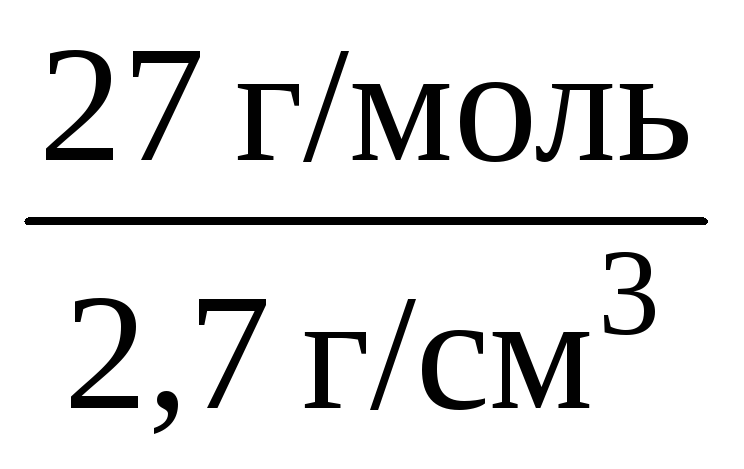 = 10 cm 3 / mol = 0.01 L / mol.
= 10 cm 3 / mol = 0.01 L / mol.













