Nitric acid decomposes into. §27. Nitric acid
Nitric acid page 3
Oxidative properties of nitric acid page 3
Nitrates page 6
Industrial production of nitric acid page 7
The cycle of nitrogen in nature p. 8
6. Bibliography page 10
1. Nitric acid. Pure nitric acid is a HNO-colorless liquid with a density of 1.51 g / cm at -42 ° C solidifying into a clear crystalline mass. In air, it, like concentrated hydrochloric acid, "smokes", as its vapors form with the "moisture of the air, small droplets of fog,
Nitric acid is not distinguished by its strength. Even under the influence of light, it gradually decomposes:
The higher the temperature and the more concentrated the acid, the faster decomposition proceeds. The liberated nitrogen dioxide dissolves in the acid and gives it a brown color.
Nitric acid is one of the most powerful acids; in dilute solutions, it completely decomposes into H and-NO ions.
2. Oxidizing properties of nitric acid. A characteristic property of nitric acid is its pronounced oxidizing ability. Nitric acid is one of the most energetic oxidants. Many non-metals are easily oxidized by it, turning into the corresponding acids. So, sulfur at boiling with nitric acid is gradually oxidized to sulfuric acid, phosphorus - to phosphoric acid. Smoldering coal, immersed in concentrated HNO, brightly flares.
Nitric acid acts on almost all metals (except gold, platinum, tantalum, rhodium, iridium), converting them into nitrates, and some metals into oxides.
Concentrated HNO passivates some metals. Lomonosov also discovered that iron, which dissolves readily in dilute nitric acid, does not dissolve in cold concentrated HNO. Later it was found that a similar action of nitric acid has on chromium and aluminum. These metals pass under the action of concentrated nitric acid into a passive state.
The degree of oxidation of nitrogen in nitric acid is 4-5. Acting as an oxidant, HNO  can be restored to various products:
can be restored to various products:
Which of these substances is formed, that is, how much nitric acid is reduced in one or another way, depends on the nature of the reducing agent and on the reaction conditions, primarily on the acid concentration. The higher the concentration of HNO, the less deeply it is restored. When reactions with concentrated acid are most often released  . In the reaction of dilute nitric acid with low-activity metals, for example, with copper, NO. In the case of more active metals - iron, zinc, - is formed
. In the reaction of dilute nitric acid with low-activity metals, for example, with copper, NO. In the case of more active metals - iron, zinc, - is formed 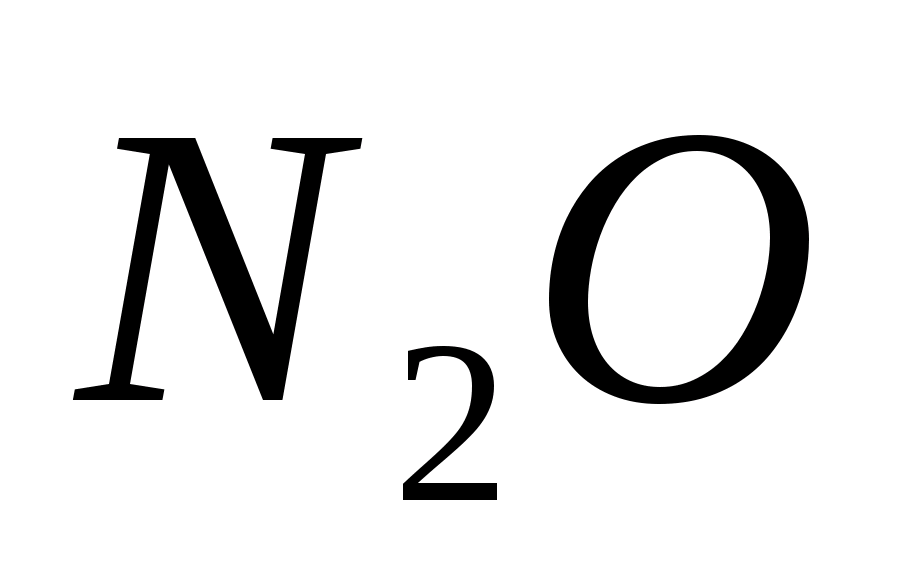 . Strongly diluted nitric acid interacts with active metals --- zinc, magnesium, aluminum - to form an ammonium ion, giving ammonium nitrate with acid. Usually several products are formed simultaneously.
. Strongly diluted nitric acid interacts with active metals --- zinc, magnesium, aluminum - to form an ammonium ion, giving ammonium nitrate with acid. Usually several products are formed simultaneously.
For illustration, let us present the schemes of oxidation reactions of some metals with nitric acid;
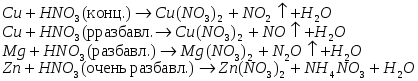
When nitric acid acts on metals, hydrogen, as a rule, is not released.
In the oxidation of nonmetals, concentrated nitric acid, as in the case of metals, is reduced to  , eg
, eg
The more dilute acid is usually reduced to NO, for example:
The illustrated schemes illustrate the most typical cases of the interaction of nitric acid with metals and nonmetals. In general, oxidation-reduction reactions involving  , flow is difficult.
, flow is difficult.
A mixture consisting of 1 volume of nitric and 3-4 volumes of concentrated hydrochloric acid is called royal vodka. Royal vodka dissolves some metals that do not interact with nitric acid, including the "king of metals" -gold. Its action is explained by the fact that nitric acid oxidizes hydrochloric acid with liberation of free chlorine and formation of nitrogen Chloridea (III), or nitrosyl chloride,  :
:
Nitrosyl chloride is an intermediate reaction product and decomposes:

Chlorine at the time of separation consists of atoms, which determines the high oxidizing ability of tsar's vodka. The oxidation reactions of gold and platinum proceed mainly according to the following equations.
With an excess of hydrochloric acid, gold chloride (III) and platinum (IV) chloride form complex compounds
On many organic substances, nitric acid acts in such a way that one or more hydrogen atoms in the molecule of an organic compound are replaced by nitro groups. This process is called nitration and is of great importance in organic chemistry.
Nitric acid is one of the most important nitrogen compounds: it is consumed in large quantities in production, nitrogen fertilizers, explosives and organic dyes, serves as an oxidant in many chemical processes, is used in the production of sulfuric acid by the nitrosic method, is used for the production of cellulose varnishes and film.
3. Nitrates. Nitric acid salts are called nitrates. All of them are readily soluble in water, and decompose when heated with the release of oxygen. The nitrates of the most active metals are converted into nitrites:
Nitrates of most other metals under heating decompose into metal oxide, oxygen and nitrogen dioxide. For example:
Finally, nitrates of the least active metals (for example, silver, gold) decompose on heating to a free metal:
Easily splitting off oxygen, nitrates at high temperature are energetic oxidants. Their aqueous solutions, on the other hand, show almost no oxidative properties.
The most important are nitrates of sodium, potassium, ammonium and calcium, which in practice are called nitrates.
Sodium nitrate
 or sodium nitrate,sometimes also called chilean nitrate, found in large numbers in nature only in Chile.
or sodium nitrate,sometimes also called chilean nitrate, found in large numbers in nature only in Chile.
Potassium Nitrate  , or potassium nitrate, in small quantities is also found in nature, but mainly obtained artificially by the interaction of sodium nitrate with potassium chloride.
, or potassium nitrate, in small quantities is also found in nature, but mainly obtained artificially by the interaction of sodium nitrate with potassium chloride.
Both these salts are used as fertilizers, and potassium nitrate contains two elements necessary for plants: nitrogen and potassium. Nitrates of sodium and potassium are also used in the glass industry and in the food industry for canning products.
Calcium nitrate
 or calcium nitrate, is obtained in large amounts by neutralizing nitric acid with lime; is used as a fertilizer.
or calcium nitrate, is obtained in large amounts by neutralizing nitric acid with lime; is used as a fertilizer.
4. Industrial production of nitric acid. Modern industrial methods for the production of nitric acid are based on the catalytic oxidation of ammonia by air oxygen. When "describing the properties of ammonia it was indicated that it burns in oxygen, and the reaction products are water and free nitrogen. But in the presence of catalysts - the oxidation of ammonia with oxygen can proceed differently. If a mixture of ammonia and air is passed over the catalyst, at 750 ° C and a certain composition of the mixture, an almost complete conversion
Formed  easily passes into, which with water in the presence of atmospheric oxygen gives nitric acid.
easily passes into, which with water in the presence of atmospheric oxygen gives nitric acid.
Alloys based on platinum are used as catalysts in the oxidation of ammonia.
The nitric acid produced by the oxidation of ammonia has a concentration not exceeding 60%. If necessary, it is concentrated,
The industry produces diluted nitric acid with a concentration of 55, 47 and 45%, and concentrated 98 and 97%, Concentrated acid is transported in aluminum tanks, diluted in acid-resistant steel tanks.
5. The cycle of nitrogen in nature. When decaying organic substances, a significant part of the nitrogen content is converted to ammonia, which, under the influence of nitrifying bacteria in the soil, is then oxidized to nitric acid. The latter, reacting with carbonates in the soil, for example calcium carbonate  , forms nitrates:
, forms nitrates:
Some of the nitrogen is always released when rotting in a free form into the atmosphere. Free nitrogen is also released when burning organic substances, burning wood, coal, peat. In addition, there are bacteria that, with insufficient air, can take oxygen from nitrates, destroying them with the release of free nitrogen. The activity of these denitrifying bacteria leads to the fact that part of the nitrogen from the form (nitrates) accessible to green plants passes into inaccessible (free nitrogen). Thus, not all of the nitrogen that was part of the dead plants is returned to the soil; part of it is gradually released in a free form.
The continuous loss of mineral nitrogen compounds would have long ago led to a complete cessation of life on Earth if there were no processes in nature that compensated for nitrogen losses. Such processes include, first of all, electrical discharges occurring in the atmosphere, under which a certain amount of nitrogen oxides is always formed; the latter with water give nitric acid, which turns in the soil into nitrates. "Another source of replenishment of nitrogen compounds of the soil is the vital activity of the so-called Azotobacteria, capable of assimilating atmospheric nitrogen, Some of these bacteria settle on the roots of plants from the legume family, causing the formation of characteristic swellings -" nodules ", why they are called nodule bacteria. , nodule bacteria process it into nitrogen compounds, and the plants, in turn, convert the latter into proteins and other complex substances. Synthesis and analysis of HCV in pro nitrate production acids Anhydrous nitric acid (monohydrate ...
Receiving nitric acids
Term paper \u003e\u003e ChemistryWith education nitric acids. Nitrogen acid is a low-stable compound and breaks up into nitric acid, Nitric oxide... nitric acid. Nitrogen gases are separated from the condensed nitric acids in the separator 8, from which nitric acid ...
- Nitric acid page 3 Oxidizing properties of nitric acid page 3 Nitrates page 6 Industrial production of nitric acid page 7 The nitrogen cycle in nature page 8
The higher the temperature and the more concentrated the acid, the faster decomposition proceeds. The liberated nitrogen dioxide dissolves in the acid and gives it a brown color. Nitric acid is one of the strongest acid kits; in dilute solutions, it completely decomposes into H and-NO ions. 2. Oxidizing properties of nitric acid. A characteristic property of nitric acid is its pronounced oxidizing ability. Nitric acid is one of the most energetic oxidants. Many non-metals are easily oxidized by it, turning into the corresponding acids. So, sulfur at boiling with nitric acid is gradually oxidized to sulfuric acid, phosphorus - to phosphoric acid. Smoldering coal, immersed in concentrated HNO, brightly flares. Nitric acid acts on almost all metals (except gold, platinum, tantalum, rhodium, iridium), converting them into nitrates, and some metals into oxides. Concentrated HNO passivates some metals. Lomonosov also discovered that iron, which dissolves readily in dilute nitric acid, does not dissolve in cold concentrated HNO. Later it was found that a similar action of nitric acid has on chromium and aluminum. These metals pass under the action of concentrated nitric acid into a passive state. The degree of oxidation of nitrogen in nitric acid is 4-5. Acting as an oxidant, HNO  can be restored to various products:
can be restored to various products:
Which of these substances is formed, that is, how much nitric acid is reduced in one or another way, depends on the nature of the reducing agent and on the reaction conditions, primarily on the acid concentration. The higher the concentration of HNO, the less deeply it is restored. When reactions with concentrated acid are most often released  . In the reaction of dilute nitric acid with low-activity metals, for example, with copper, NO. In the case of more active metals - iron, zinc, - is formed
. In the reaction of dilute nitric acid with low-activity metals, for example, with copper, NO. In the case of more active metals - iron, zinc, - is formed  . Strongly diluted nitric acid interacts with active metals --- zinc, magnesium, aluminum - to form an ammonium ion giving ammonium nitrate with acid. Usually, several products are formed simultaneously. To illustrate, we give the schemes of the reactions of the oxidation of certain metals with nitric acid;
. Strongly diluted nitric acid interacts with active metals --- zinc, magnesium, aluminum - to form an ammonium ion giving ammonium nitrate with acid. Usually, several products are formed simultaneously. To illustrate, we give the schemes of the reactions of the oxidation of certain metals with nitric acid;
![]()
Under the action of nitric acid on metals, hydrogen, as a rule, is not released. In the oxidation of non-metals, concentrated nitric acid, as in the case of metals, is reduced to  , eg
, eg
The more dilute acid is usually reduced to NO, for example:
The illustrated schemes illustrate the most typical cases of the interaction of nitric acid with metals and nonmetals. In general, oxidation-reduction reactions involving  , flow is difficult. A mixture consisting of 1 volume of nitric and 3-4 volumes of concentrated hydrochloric acid is called royal vodka. Royal vodka dissolves some metals that do not interact with nitric acid, including the "king of metals" -gold. Its operation is explained by the fact that nitric acid oxidizes hydrochloric acid with liberation of free chlorine and formation of nitrogen Chloridea (III), or nitrosyl chloride,
, flow is difficult. A mixture consisting of 1 volume of nitric and 3-4 volumes of concentrated hydrochloric acid is called royal vodka. Royal vodka dissolves some metals that do not interact with nitric acid, including the "king of metals" -gold. Its operation is explained by the fact that nitric acid oxidizes hydrochloric acid with liberation of free chlorine and formation of nitrogen Chloridea (III), or nitrosyl chloride,  :
:
Nitrosyl chloride is an intermediate reaction product and decomposes:

Chlorine at the time of separation consists of atoms, which determines the high oxidizing ability of tsar's vodka. The oxidation reactions of gold and platinum proceed mainly in accordance with the following equations.
With an excess of hydrochloric acid, gold chloride (III) and platinum chloride (IV) form complex compounds. Many organic substances act nitric acid so that one or more hydrogen atoms in the molecule of the organic compound are replaced by nitro groups. This process is called nitration and is of great importance in organic chemistry. Nitric acid is one of the most important compounds of nitrogen: in large quantities it is consumed in production, nitrogen fertilizers, explosives and organic dyes, is an oxidizer in many chemical processes, is used in the production of sulfuric acid by the nitrosic process, is used for the production of cellulose varnishes, film. 3. Nitrates. Nitric acid salts are called nitrates. They all dissolve well in water, and decompose with heating with oxygen. The nitrates of the most active metals are converted into nitrites:
Nitrates of most other metals under heating decompose into metal oxide, oxygen and nitrogen dioxide. For example:
Finally, nitrates of the least active metals (eg, se-ribs, gold) decompose on heating to free metal:
Easily splitting off oxygen, nitrates at high temperature are energetic oxidants. Their aqueous solutions, on the contrary, almost do not exhibit oxidizing properties. The most important are nitrates of sodium, potassium, ammonium and calcium, which in practice are called nitrates. Sodium nitrate  or sodium nitrate,sometimes also called chilean nitrate, found in a large number in nature only in Chile. Potassium Nitrate
or sodium nitrate,sometimes also called chilean nitrate, found in a large number in nature only in Chile. Potassium Nitrate  , or potassium nitrate, in small quantities is also found in nature, but mostly it is obtained artificially when sodium nitrate reacts with the chloride-potassium. Both these salts are used as fertilizers, and potassium nitrate contains two elements necessary for plants: nitrogen and potassium. Nitrates of sodium and potassium are also used in glass-making and in the food industry for canning produktov. Calcium nitrate
, or potassium nitrate, in small quantities is also found in nature, but mostly it is obtained artificially when sodium nitrate reacts with the chloride-potassium. Both these salts are used as fertilizers, and potassium nitrate contains two elements necessary for plants: nitrogen and potassium. Nitrates of sodium and potassium are also used in glass-making and in the food industry for canning produktov. Calcium nitrate 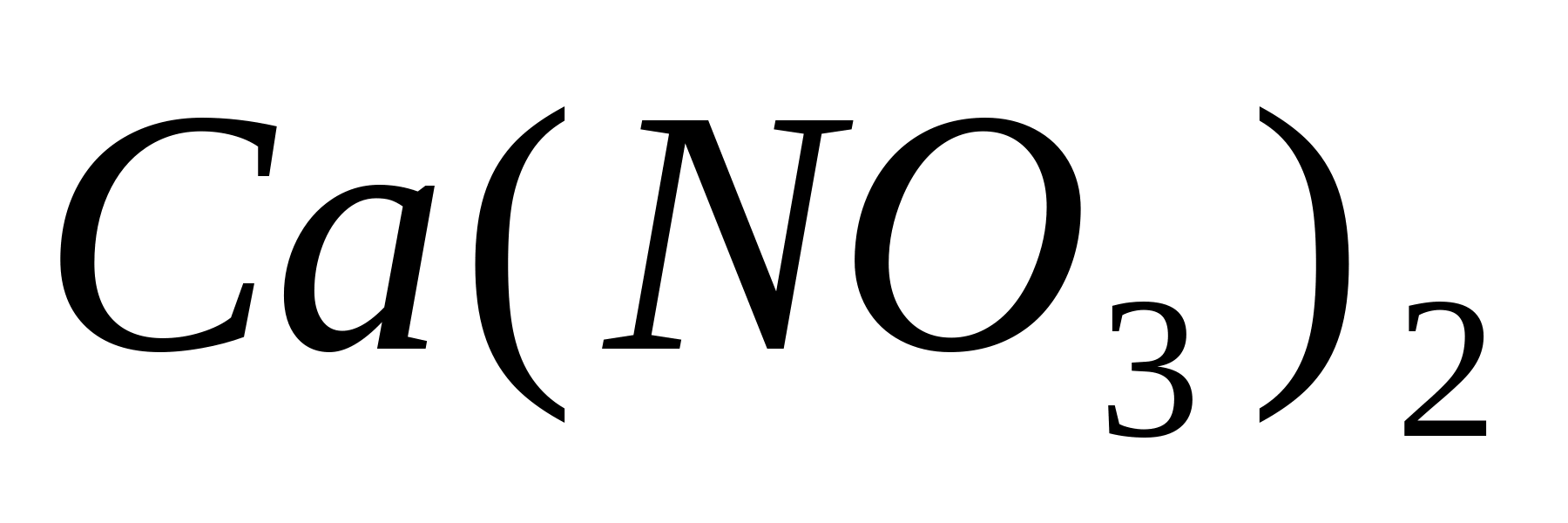 or calcium nitrate, is obtained in large amounts by neutralizing nitric acid with lime; is used as a fertilizer. 4. Industrial production of nitric acid. Modern industrial methods for the production of nitric acid are based on the catalytic oxidation of ammonia by air oxygen. When "describing the properties of ammonia it was indicated that it burns in oxygen, and the reaction products are water and free nitrogen. But in the presence of catalysts - the oxidation of ammonia with oxygen can proceed differently. If a mixture of ammonia and air is passed over the catalyst, then at 750 ° C and a certain composition of the mixture, an almost complete conversion
or calcium nitrate, is obtained in large amounts by neutralizing nitric acid with lime; is used as a fertilizer. 4. Industrial production of nitric acid. Modern industrial methods for the production of nitric acid are based on the catalytic oxidation of ammonia by air oxygen. When "describing the properties of ammonia it was indicated that it burns in oxygen, and the reaction products are water and free nitrogen. But in the presence of catalysts - the oxidation of ammonia with oxygen can proceed differently. If a mixture of ammonia and air is passed over the catalyst, then at 750 ° C and a certain composition of the mixture, an almost complete conversion
Formed  easily passes into, which with water in the presence of atmospheric oxygen gives nitric acid. Alloys based on platinum are used as catalysts in the oxidation of ammonia. The nitric acid produced by the oxidation of ammonia has a concentration not exceeding 60%. If necessary, concentrate it, Industry produces diluted nitric acid with a concentration of 55, 47 and 45%, and concentrated 98 and 97%, Concentrated acid is transported in aluminum tanks, diluted in acid-resistant steel tanks. 5. The cycle of nitrogen in nature. When decay of organic substances, a significant part of the nitrogen content is converted to ammonia, which, under the influence of nitrifying bacteria in the soil, is then oxidized to nitric acid. The latter, reacting with carbonates in the soil, for example calcium carbonate
easily passes into, which with water in the presence of atmospheric oxygen gives nitric acid. Alloys based on platinum are used as catalysts in the oxidation of ammonia. The nitric acid produced by the oxidation of ammonia has a concentration not exceeding 60%. If necessary, concentrate it, Industry produces diluted nitric acid with a concentration of 55, 47 and 45%, and concentrated 98 and 97%, Concentrated acid is transported in aluminum tanks, diluted in acid-resistant steel tanks. 5. The cycle of nitrogen in nature. When decay of organic substances, a significant part of the nitrogen content is converted to ammonia, which, under the influence of nitrifying bacteria in the soil, is then oxidized to nitric acid. The latter, reacting with carbonates in the soil, for example calcium carbonate  , forms nitrates:
, forms nitrates:
Some of the nitrogen is always released when rotting in a free form into the atmosphere. Free nitrogen is also released when burning organic substances, burning wood, coal, peat. In addition, there are bacteria that, if air is insufficiently available, can take oxygen away from nitrates, destroying them with the release of free nitrogen. The activity of these denitrifying bacteria leads to the fact that part of the nitrogen from the form (nitrates) accessible to green plants passes into inaccessible (free nitrogen). Thus, not all of the nitrogen that was part of the dead plants is returned to the soil; part of it is gradually released in a free form. The continuous loss of mineral nitrogen compounds would have long ago led to a complete cessation of life on Earth, if there were no processes in nature that compensated for nitrogen losses. Such processes include, first of all, electric discharges in the atmosphere, during which a certain amount of nitrogen oxides is formed; the latter with water give nitric acid, which turns in the soil into nitrates. "Another source of replenishment of nitrogen compounds of the soil is the vital activity of so-called Azotobacteria, capable of assimilating atmospheric nitrogen, Some of these bacteria settle on the roots of plants from the legume family, causing the formation of characteristic swellings -" nodules ", why they are called nodule bacteria. atmospheric nitrogen, nodule bacteria convert it to nitrogen compounds, and plants, in turn, convert the latter into proteins and other complex substances. Thus, in nature The continuous circulating nitrogen of nitrogen is terminated, however, the most protein-rich parts of plants, for example grain, are annually harvested from the fields, so fertilizers must be added to the soil to compensate for the loss of the most important plant nutrients. application of fertilizers is the subject of a special branch of chemistry, called agrochemistry.
1. Explain why nitric acid is referred to as oxidizing acids. What other oxidizing acids are known to you? Is it possible to classify hydrochloric acid with such acids? Discuss this question with your neighbor on the school desk.
In nitric acid, the degree of oxidation of nitrogen +5 (the highest positive oxidation state), so it easily takes electrons and is an oxidizing agent. Similar oxidizing acids: H₂SO₄, HCl₄₄, H₂C₂rO₇. In hydrochloric acid, the degree of oxidation of chlorine-1 (the smallest negative), it can no longer receive electrons and be an oxidizer.
2. In the reaction of dilute nitric acids with certain metals (Mg, Zn), nitric oxide (I) or nitrogen can be released. Write the appropriate equations of oxidation-reduction reactions.
3. Write molecular, complete ionic and reduced ionic equations of reactions, with which you can distinguish between hydrochloric, sulfuric and nitric acids. 
4. Give the reaction equations: a) characteristic only for nitric acid; b) common for nitric and other acids. 
5. Write the equations for the reactions between silver and diluted, as well as concentrated nitric acid. Show the transition of electrons and emphasize the oxidizer with one line, and the reductant with two. 
6. Using figure 26, prepare a computer presentation on the topic "Application of nitric acid". 
7 *. What volume of ammonia (nu) is required to produce 50 tons of solution with a mass fraction of nitric acid 0.5? 
TEST TASKS
1. Establish a correspondence between the starting materials and reaction products.
1) HNO₃ → NO₂ + O₂ + H₂O
2) NH₄NO₃ + KOH → NH₃ + KNO₃ + H₂O
3) Cu (NO₃) ₂ + KOH → Cu (OH) ₂ ↓ + KNO₃
4) Zn (NO₃) ₂ (TV) + H₂SO₄ (conc.) → HNO₃ + ZnSO₄
2. Nitric acid does not interact
1) with carbon monoxide (IV)
Oxidizing properties of nitric acid. A characteristic property of nitric acid is its pronounced oxidizing ability.
Nitric acid is one of the most energetic oxidants. Many non-metals are easily oxidized by it, turning into the corresponding acids. So, sulfur at boiling with nitric acid is gradually oxidized to sulfuric acid, phosphorus to phosphoric acid. Smoldering coal, immersed in concentrated HNO, brightly flares. Nitric acid acts on almost all metals except gold, platinum, tantalum, rhodium, iridium, converting them into nitrates, and some metals into oxides.
Concentrated HNO passivates some metals. Lomonosov also discovered that iron, which dissolves readily in dilute nitric acid, does not dissolve in cold concentrated HNO. Later it was found that a similar action of nitric acid has on chromium and aluminum. These metals pass under the action of concentrated nitric acid into a passive state.
The degree of oxidation of nitrogen in nitric acid is 4-5. Acting as an oxidizer, HNO can be reduced to various products. Which of these substances is formed, that is, how much nitric acid is reduced in one or another way, depends on the nature of the reducing agent and on the reaction conditions, primarily on the acid concentration. The higher the concentration of HNO, the less deeply it is restored. When reactions with concentrated acid are most often released. In the reaction of dilute nitric acid with low-activity metals, for example, with copper, NO is released. In the case of more active metals, iron, zinc, is formed. Strongly diluted nitric acid interacts with active metals with zinc, magnesium, and aluminum to form an ammonium ion, which gives ammonium nitrate with acid.
Usually several products are formed simultaneously. To illustrate, we give the schemes of oxidation reactions of some metals with nitric acid. When nitric acid acts on metals, hydrogen, as a rule, is not released.
In the oxidation of nonmetals, concentrated nitric acid, as in the case of metals, is reduced to, for example. The more dilute acid is usually reduced to NO, for example. The illustrated schemes illustrate the most typical cases of interaction of nitric acid with metals and nonmetals. In general, the oxidation-reduction reactions that occur with participation are difficult. A mixture consisting of 1 volume of nitric and 3 4 volumes of concentrated hydrochloric acid is called royal vodka.
Royal vodka dissolves some metals that do not interact with nitric acid, including the gold king. Its action is explained by the fact that nitric acid oxidizes hydrochloric acid with the liberation of free chlorine and the formation of chlorine oxide of nitrogen or nitrosyl chloride, nitrosyl chloride is an intermediate product of the reaction and decomposes. Chlorine at the time of separation consists of atoms, which determines the high oxidizing ability of tsar's vodka.
The oxidation reactions of gold and platinum proceed mainly according to the following equations. With an excess of hydrochloric acid, gold chloride III and platinum chloride IV form complex compounds. For many organic substances, nitric acid acts in such a way that one or more hydrogen atoms in the molecule of the organic compound are replaced by nitro groups. This process is called nitration and is of great importance in organic chemistry. Nitric acid is one of the most important nitrogen compounds in large quantities, it is used in the production of nitrogen fertilizers, explosives and organic dyes, serves as an oxidant in many chemical processes, is used in the production of sulfuric acid by the nitrosic method, is used for the production of cellulose varnishes and film. 3. Nitrates. Nitric acid salts are called nitrates. All of them are readily soluble in water, and decompose when heated with the release of oxygen.
The nitrates of the most active metals are converted to nitrites. The nitrates of most other metals decompose on metal oxide, oxygen and nitrogen dioxide when heated.
For example, Finally, nitrates of the least active metals such as silver, gold decompose on heating to a free metal. By easily eliminating oxygen, nitrates at high temperature are energetic oxidants. Their aqueous solutions, on the other hand, show almost no oxidative properties. The most important are nitrates of sodium, potassium, ammonium and calcium, which in practice are called nitrates.
Sodium nitrate or sodium nitrate, sometimes also called Chilean nitrate, is found in large quantities in nature only in Chile. Potassium nitrate, or potassium nitrate, is also found in small amounts in nature, but is mainly obtained artificially by the interaction of sodium nitrate with potassium chloride. Both these salts are used as fertilizers, and potassium nitrate contains two elements necessary for plants: nitrogen and potassium.
Nitrates of sodium and potassium are also used in the glass industry and in the food industry for canning products. Calcium nitrate or calcium nitrate, obtained in large amounts by neutralizing nitric acid with lime is used as a fertilizer. 4.
End of work -
This topic belongs to the section:
Nitric acid
The liberated nitrogen dioxide dissolves in the acid and gives it a brown color. Nitric acid is one of the strongest acids in ... Nitric acid is one of the most energetic oxidants. Many non-metals are easy ... Concentrated HNO passivates some metals. Lomonosov also discovered that iron, which dissolves easily in ...
If you need additional material on this topic, or you did not find what you were looking for, we recommend you use the search on our database:
What we will do with the material:
If this material turned out to be useful for you, you can save it on your page in social networks: